The feasibility of electromagnetic navigational bronchoscopic localization with fluorescence and radiocontrast dyes for video-assisted thoracoscopic surgery resection
Introduction
In recent years, lung segmentectomy or wedge resection has been developed to offer better pulmonary preservation than lobectomy or pneumonectomy for lung malignancy (1,2). Sublobar resection has been used for the removal of inflammatory lung lesions or non-malignant lung masses (3,4); meanwhile, for early-stage lung malignancy (5-7), some groups have recommended the use of localization techniques to achieve a proper resection margin of more than 2 cm or the size of nodule (8-10).
Lobectomy remains the gold standard of treatment for early-stage lung cancer. Sometimes, we consider a sublobar resection in patients with poor cardiopulmonary reserve, which could be threatened by conventional lobectomy; another indication is preservation of normal pulmonary reserve in patients with very small lung malignancies. This corresponds to peripheral nodules smaller than 2 cm with a pure adenocarcinoma in-situ histology, more than 50% ground-glass portion on computed tomography (11) scan, and a long doubling time (>400 days) on radiologic imaging surveillance (12-14). In these cases, segmentectomy is preferred over wedge resection because of the high incidence of local recurrence with a wedge resection (15).
Further challenges are created by the single-port (16,17), subxiphoid (18) and robotic approaches (19,20) in lung cancer surgery. Technical issues regarding video-assisted thoracoscopic surgery (VATS) approaches for segmentectomy might be summarized as identification of the correct location of the target lesion and the proper sufficient resection margin for control of local recurrence (21,22). During open thoracotomy, surgeons can perform manual palpation of the target lesion and directly inspect the anatomic landmark for the intersegmental plane. On the other hand, the operative field might be limited for manual palpation and delineation of the intersegmental plane or for the identification of surgical anatomic landmarks. Thus, preoperative localization techniques were introduced to help locate non-palpable or deep-seated nodules at the time of VATS. Dyes such as methylene blue (23,24) and indigo carmine (25,26), radiocontrast (lipiodol or iopamidol) (27,28), metallic markers such as hook-wire (29) or fiducials (30), and radioisotopes (31,32) were used for locating the lesions. These techniques, however, require preoperative percutaneous transthoracic intervention with CT scan, and occasionally, these techniques might be harmful for patients, as they may develop complications, particularly pneumothorax, hemothorax or embolization.
Recently, electromagnetic navigational bronchoscopy (ENB) has been applied to help localize the target lesion prior to surgical resection (26). ENB utilizes electromagnetic technology to guide the bronchoscope and the catheter using a three-dimensional (3D) virtual map from thin-section CT scan images. Historically, ENB was developed as a minimally invasive diagnostic tool for detection of lung lesions. The system consisted of a computer software for three-dimensional CT reconstruction used for planning, a positional sensor and magnetic generator in bed, and a navigational system for bronchoscopic guidance. For diagnostic purposes, ENB showed similar or superior diagnostic yield with regard to peripheral lung lesions compared to another diagnostic modality (transthoracic needle aspiration), with less procedure-related complications (33,34).
Experience of ENB localization for VATS sublobar resection
ENB was first introduced in our center in 2017 and we used ENB localization with fluorescent dye for VATS or robotic-assisted sublobar resection in ten patients. The superDimension system version 7 (Medtronic, Minneapolis, MN, USA) was used in our clinical experience. During that time, we concurrently performed multiple localization techniques for VATS or RATS resection, using CT-guided transthoracic injection of indocyanine green (ICG) with lipiodol, and hook-wire localization with or without lipiodol. We used the ICG dye for ENB localization with an endoscopic fluorescence imaging system (Pinpoint; Novadaq, Kalamazoo, MI, USA), which uses near infrared (NIR) imaging, for VATS. Additional lipiodol was mixed to the ICG dye to evaluate the surgical resection margin and to identify the appropriate intersegmental plane during sublobar resection (Figure 1).
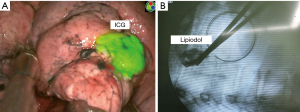
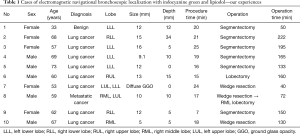
In our study population (n=10), six patients underwent planned VATS segmentectomy for whom we performed intraoperative ENB localization with ICG and lipiodol to evaluate the location of the nodule and the appropriate intersegmental plane. Three patients underwent planned VATS wedge resection for an indeterminate pulmonary nodule without tissue diagnosis. The last patient had interstitial lung disease with mixed ground glass opacity (GGO) on CT scan, in whom we performed ENB localization to determine the accurate location of areas with higher malignant potential (Table 1).
Full table
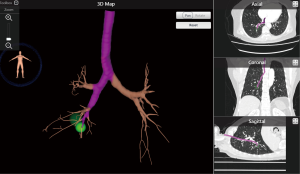
For ENB localization with dye injection, we first need a CT scan with thin-section images to reconstruct a 3D map of the virtual airway and multiplanar views to create the pathway to the target lesion (Figure 2).
A 3D reconstruction software embedded on a laptop system was used to generate 3D mapping. In the operating room, the location board was placed on the operative bed to create an electromagnetic field and magnetic sensors for tracking were placed on the patient’s anterior chest wall to show the real-time position of the locatable catheter tip.
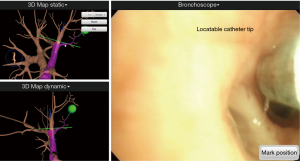
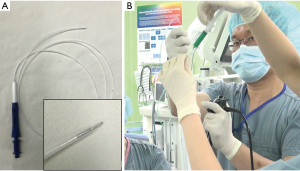
We then performed endotracheal (ET) intubation with a single lumen ET tube with an 8.0 internal diameter using a 6-mm bronchoscope, BF-1T260 with a 2.8-mm working channel (Olympus, Tokyo, Japan) for the ENB localization. A steerable navigation catheter with a location sensor tip was inserted in the working channel of the bronchoscope. We then inserted the bronchoscope to examine the airway and proceeded with the registration sequence to synchronize the virtual and actual anatomies of the patient (Figure 3).
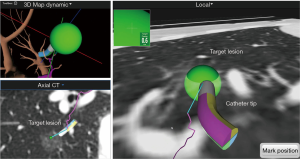
After registration was completed, the main monitor began to show the virtual bronchoscopic view on the screen. As the bronchoscope was advanced to the target lesion, the navigation catheter was wedged into the subsegmental bronchus. From this point, a locator guide was moved toward the target, and the real-time location of the catheter tip was displayed on the monitor. The navigational catheter could approach the target lesion by rotating and advancing along the pathway generated through virtual bronchoscopic images. The target lesion appeared as a green sphere on the monitor and the distance from the catheter tip was simultaneously displayed (Figure 4). Once the catheter tip reached the target lesion, the catheter was fixed, and the locatable sensor tip removed. We then inserted a 25-gauge injection needle (diameter 1.9 mm) which has sufficient length (1.65 m) to reach the peripheral lesion through the catheter, and we injected 1 ml of mixed ICG dye and lipiodol (Figure 5).
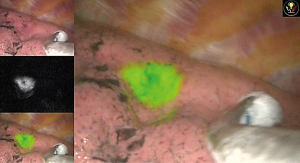
To evaluate the real-time diffusion pattern of the injected dye, C-arm fluoroscopy was used to confirm the dye diffusion After the ENB localization with dye was completed, we removed the bronchoscope and changed the position of the patient to lateral decubitus for VATS or robotic resection. The mean procedure time was 18.2 minutes in our study. We used the endoscopic fluorescence imaging system for VATS and the SPY imaging system (Figure 6) embedded on the Da Vinci robotic system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) during robotic lung resection. The outcomes are summarized in Table 1. The success rate was 90% and we failed only in a single case: the pure GGO lesion which was located 34-mm deep from the pleura. In this case, we planned the target location directly within the GGO lesion; however, we could not see the ICG signal on intraoperative SPY images. The ICG signal appeared after dissection deep in the lung segment. Of the nine malignancies, we achieved clear resection margins in eight cases for segmentectomy or wedge resection. We converted to VATS lobectomy in one case because the resection margin was positive on the intersegmental plane which fluorescent signal detected on fluorescent camera and we confirmed the positive margin on frozen biopsy. Minor endobronchial bleeding occurred during ENB localization, which stopped with compression. There was no adverse systemic event related with the ENB localization and dye injection.
Role of electromagnetic navigational bronchoscopy prior to VATS sublobar resection
There have been many attempts on localization of small pulmonary lesions to aid in improving visual identification and guidance for resection. Ideally, there is no need for devices and additional cost if the target lesion can be palpated by finger. However, for VATS sublobar resection without localization, 10–20% of cases have been reported to have missed nodules or have failed to achieve sufficient resection margins (35,36). Although percutaneous localization with dye (methylene blue) showed more than 80–90% success rate in published series (23,37), there have been unsolved problems, such as limited time interval between dye injection and operation (within 3 hours) to minimize dye diffusion, and lack of information about the accurate depth of the resection margin. CT-guided localization using hook wire or microcoils (38,39), radiolabeled aggregates (40,41), and lipiodol (42,43) also showed approximately 90–100% success rate with acceptable outcomes in randomized trials; however, these procedures are associated with a risk of procedure-related complications, namely pneumothorax, hemothorax, or for lipiodol, embolism. Microcoils or hook wires might also be displaced or dislodged in about 2–10% of cases (44). These localization procedures requisitely need the help of a radiologist or nuclear medicine specialist. Intraoperative ultrasonography might be a good option for identification of a solid pulmonary lesion, with a 93% success rate (45). However, the success of this technique depends on the surgeon’s experience and the results may not be accurate if the lung is emphysematous (46). In addition, with the development of 3D multi-detector CT, some groups have used 3D reconstruction with different color mapping for arteries and veins, and lung segmental anatomy for surgical planning (47,48). This image-guided technique with 3D reconstruction might be helpful in the performance of lung segmentectomy, however, this has not been widely adapted at present.
The available evidence supporting the clinical efficacy of ENB as a localization tool for surgical resection is currently weak, with no randomized trials. However, the use of the ENB has been increasing, with the potential benefits of simplifying the current localization procedure, since this may be directly performed by the surgeon in the operating room, as well as extremely low rates of procedure-related complications. Multiple groups have described the use of ENB as a localization tool with methylene blue or ICG for the resection of lung nodules. Bolton et al. used ENB with methylene blue dye for localization of lung nodules in 19 patients who underwent robotic surgery (26). The mean tumor size was 18 (range, 8–40) mm and procedure time was 28 (range, 17–40) minutes. Using this method, they reported a 100% success rate, with no adverse event or conversion to open thoracotomy. Krimsky et al. also reported on ENB localization with methylene blue and ICG dye in 21 patients (49). The mean tumor size was 13.4 (range, 7–29) mm and ICG dye was used in ten patients. The success rate was 81% (17/21); there was no visible dye in three cases and the dye extravasated in one case, which made the mass impossible to identify. Marino et al. reported the largest case series using methylene blue dye for ENB localization in 70 patients (50). The mean tumor size was 8 (range, 4–17) mm and the mean distance from the pleura was 6 (range, 1–19) mm. By using this method, they reported a 97.2% success rate, with two cases of dye extravasation. Awais et al. also described the use of ENB localization using methylene blue in 29 patients (51). They performed 33 localization procedures for lung lesions with a mean nodule size of 10 (range, 4–27) mm and depth from the pleura of 13 (range, 3–44) mm. They reported a 100% success rate with the use of additional intraoperative CT scan to achieve adequate surgical resection margins. Abbas et al. used ENB localization with methylene blue, either alone or with ICG and iopamidol, or fiducial marker in two cases (28). They performed VATS resection in two patients and robotic resection in 47 patients. Two patients required thoracotomy for severe adhesions. They used additional ICG to use the NIR SPY images by robotic endoscope during the procedure. Iopamidol was also used to inspect the real-time flow pattern of the methylene blue dye by intraoperative fluoroscopy. The success rate was 98.1% in 54 localization procedures.
As a localization tool for ENB with dye in this reported case series, ENB is a safe and feasible modality for intraoperative localization during VATS or robotic sublobar resection. The reported success rate of ENB localization ranged from 80% to 100%, but was generally more than 95% in most case series. In literature review, the success rate of ENB localization for surgical resection appears to be acceptable, compared with other localization techniques; however, this should be evaluated in large series or randomized studies (Table 2).

Full table
There was no adverse systemic event related with bronchoscopic dye injection. The ENB localization technique for surgical resection has potential benefits compared to the current established localization techniques. Transthoracic procedures such as CT-guided injection of marker, metallic hook wire, or microcoils were associated with development of pneumothorax, hemothorax, displacement of metallic markers, embolism, and parenchymal hematoma. Furthermore, there have been safety concerns with regard to the time delay from the localization procedure to the operation, and discomfort and inconveniences for the patients with an additional procedure. Usually, ENB localization could be performed directly in the operating room by the surgeon or the bronchoscopist, with or without ET intubation under general anesthesia. In addition, we could proceed with the planned operation without transport of the patient, which means this technique could minimize the waiting time between localization and operation to within 10 to 20 minutes, enabling the surgeon to use rapidly diffusible dyes such as methylene blue. However, ENB localization with dye injection alone might not be useful for target lesions in deep locations. For the deeply-seated pulmonary lesion, the usual target location of CT planning should be placed at the nearest pleura to visualize the dye color. The penetration power of the dye might sometimes be limited if the target location is too far away from the pleura. There have been no studies regarding the optimal distance for dye injection from the pleura to the mass during ENB marking (52). In such cases, the additional mixing of radiocontrast dye and intraoperative cone beam CT or fluoroscopy might help evaluate the deep resection margin or intersegmental plane during segmentectomy. ENB-guided deployment of additional fiducial markers might also be an option.
Thus, ENB localization with dye injection might be a feasible option for minimally invasive resection of small pulmonary nodules without tissue diagnosis. This method might also help identify the location with visual identification and can be performed safely in the operating room without delay. Furthermore, ENB could minimize the procedure-related complications frequently occurring with the currently performed transthoracic localization techniques.
Summary
From our preliminary experiences of ENB localization for VATS or robotic surgical resection, the entire localization procedure could be performed in the operating room without the help of a radiologist or bronchoscopist. The ENB localization procedure did not excessively extend the operation time. With high success rates of up to 90% and few procedure-related complications frequently associated with transthoracic intervention, such as pneumothorax or hemothorax, ENB localization might be a safe and feasible localization technique in the near future. In our technique, the use of ICG could provide more colorful images compared to methylene blue, which frequently produces diffuse staining. Potentially, additional radiocontrast such as lipiodol might prevent the dye diffusion, which should be evaluated in future studies. To overcome the insufficient resection margins for curative sublobar resection or deep-seated lesions that might not be visible even with localization, intraoperative cone-beam CT or C-arm fluoroscopy with the use of radiocontrast dye or fiducial markers can be useful to identify the deep location.
Acknowledgements
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR14C0007) and (grant number: HI17C0654 ).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mentzer SJ, Myers DW, Sugarbaker DJ. Sleeve lobectomy, segmentectomy, and thoracoscopy in the management of carcinoma of the lung. Chest 1993;103:415S-417S. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg 2010;89:1597-602. [Crossref] [PubMed]
- Guo W, Zhao YP, Jiang YG, et al. Surgical treatment and outcome of pulmonary hamartoma: a retrospective study of 20-year experience. J Exp Clin Cancer Res 2008;27:8. [Crossref] [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small cT1N0 non-small cell lung cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Landreneau RJ, D'Amico TA, Schuchert MJ, et al. Segmentectomy and Lung Cancer: Why, When, How, and How Good? Semin Thorac Cardiovasc Surg 2017;29:119-28. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. Thoracoscopic wedge resection and segmentectomy for small-sized pulmonary nodules. J Vis Surg 2017;3:66. [Crossref] [PubMed]
- Han KN, Kim HK, Lee HJ, et al. Single-port video-assisted thoracoscopic pulmonary segmentectomy: a report on 30 casesdagger. Eur J Cardiothorac Surg 2016;49 Suppl 1:i42-7. [PubMed]
- Ruffini E, Parola A, Papalia E, et al. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001;20:30-6; discussion 36-7. [Crossref] [PubMed]
- Villamizar N, Swanson SJ. Lobectomy vs. segmentectomy for NSCLC (T<2 cm). Ann Cardiothorac Surg 2014;3:160-6. [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. [Crossref] [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Han KN, Kim HK, Choi YH. Midterm outcomes of single port thoracoscopic surgery for major pulmonary resection. PLoS One 2017;12:e0186857. [Crossref] [PubMed]
- Ng CS, Rocco G, Wong RH, et al. Uniportal and single-incision video-assisted thoracic surgery: the state of the art. Interact Cardiovasc Thorac Surg 2014;19:661-6. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin 2014;24:157-62. vi. [Crossref] [PubMed]
- D'Amico TA. Thoracoscopic segmentectomy: technical considerations and outcomes. Ann Thorac Surg 2008;85:S716-8. [Crossref] [PubMed]
- Sesti J, Donington JS. Sublobar Resection: Ongoing Controversy for Treatment for Stage I Non-Small Cell Lung Cancer. Thorac Surg Clin 2016;26:251-9. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Sun J, Mao X, Xie F, et al. Electromagnetic navigation bronchoscopy guided injection of methylene blue combined with hookwire for preoperative localization of small pulmonary lesions in thoracoscopic surgery. J Thorac Dis 2015;7:E652-E656. [PubMed]
- Endo M, Kotani Y, Satouchi M, et al. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest 2004;125:1747-52. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Nomori H, Horio H, Naruke T, et al. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg 2002;74:170-3. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Sugi K, Kaneda Y, Hirasawa K, et al. Radioisotope marking under CT guidance and localization using a handheld gamma probe for small or indistinct pulmonary lesions. Chest 2003;124:155-8. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Arenberg D. Electromagnetic navigation guided bronchoscopy. Cancer Imaging. 2009;9:89-95. [PubMed]
- Reynisson PJ, Leira HO, Hernes TN, et al. Navigated bronchoscopy: a technical review. J Bronchology Interv Pulmonol 2014;21:242-64. [Crossref] [PubMed]
- Parsons AM, Detterbeck FC, Parker LA. Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary? Ann Thorac Surg 2004;78:1910-6; discussion 1916-8.
- Cerfolio RJ, Bryant AS. Is palpation of the nonresected pulmonary lobe(s) required for patients with non–small cell lung cancer? A prospective study. J Thorac Cardiovasc Surg 2008;135:261-8. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Kha LC, Hanneman K, Donahoe L, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging 2016;31:15-22. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-guided localization and resection of small or Ill-defined pulmonary lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Kim KS, Beck KS, Lee KY, et al. CT localization for a patient with a ground-glass opacity pulmonary nodule expecting thoracoscopy: a mixture of lipiodol and India ink. J Thorac Dis 2017;9:E349-E353. [Crossref] [PubMed]
- Kidane B, Yasufuku K. Advances in Image-Guided Thoracic Surgery. Thorac Surg Clin 2016;26:129-38. [Crossref] [PubMed]
- Khereba M, Ferraro P, Duranceau A, et al. Thoracoscopic localization of intraparenchymal pulmonary nodules using direct intracavitary thoracoscopic ultrasonography prevents conversion of VATS procedures to thoracotomy in selected patients. J Thorac Cardiovasc Surg 2012;144:1160-5. [Crossref] [PubMed]
- Mattioli S, D'Ovidio F, Daddi N, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg 2005;79:443-9; discussion 449. [Crossref] [PubMed]
- Nakamoto K, Omori K, Nezu K, et al. Superselective segmentectomy for deep and small pulmonary nodules under the guidance of three-dimensional reconstructed computed tomographic angiography. Ann Thorac Surg 2010;89:877-83. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-S715. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014.4. [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Muñoz-Largacha JA, Ebright MI, Litle VR, et al. Electromagnetic navigational bronchoscopy with dye marking for identification of small peripheral lung nodules during minimally invasive surgical resection. J Thorac Dis 2017;9:802-8. [Crossref] [PubMed]