Electromagnetic navigation bronchoscopy under intravenous sedation—tips and tricks
Introduction
Pulmonary nodules (PNs) is an increasing diagnostic challenge, because of its increasing detection by widespread availability of computed tomography (CT) scanning, and the concern that it may be malignant in nature.
When a biopsy is necessary to obtain a diagnosis for PNs, CT-guided transthoracic needle aspiration (CT-TTBA) has traditionally been recommended because of its high diagnostic yield (~90%); however, CT-TTBA carries a relatively high risk of iatrogenic pneumothorax (up to 25%) (1,2). Several guided bronchoscopy methods are developed to diagnose PN, including electromagnetic navigation bronchoscopy (ENB), radial endobronchial ultrasound (R-EBUS) and virtual bronchoscopy (VB). Collectively they have better diagnostic yield (pool diagnostic yield 70%) over traditional flexible bronchoscopy (FB)/transbronchial biopsy (TBBx) and lower complication rate over CT-TTBA (3).
ENB is gaining acceptance and popularity amongst bronchoscopists as one of the tools to diagnose PNs. It can be used on its own, or be combined with R-EBUS to enhance the diagnostic yield (4,5). ENB can be carried out under general anaesthesia (GA) or intravenous sedation (IVS), although GA appears to be favoured by the majority of practitioners (6,7). It has been suggested that GA may reduce patient’s movement and cough, allows better alignment of the patient’s anatomy during bronchoscopy and the planning CT thorax, and thereby improves diagnostic yield. In a meta-analysis, GA was associated with better diagnostic yield (69.2% vs. 57.5% in studies with IVS, P=0.02) (6). However, in a retrospective review of 107 patients (120 target lesions), no difference in diagnostic yield was found between GA vs. IVS (8). Duration of ENB was the only significant difference between the GA (mean 58 min) vs. IVS (mean 43 min) groups (P=0.0005). The authors suggested that the time saved with ENB under IVS may allow more procedures to be performed, with greater flexibility in scheduling.
The prevalence of PNs appears to be much higher in Asia versus Europe and North America (9). On the other hand, the evaluation of PNs in Asia is complicated by the high prevalence of tuberculosis (TB) and the fact that non-smoking history does not have any weight in discounting malignancy risk (10). Therefore, an Asian consensus guideline suggests less reliance on PET scanning, surgical diagnosis or surveillance, and it is in favour of more non-surgical biopsies (e.g., CT-TTBA or guided bronchoscopy) (11). In this context, it is anticipated that the need for ENB will continue to expand in Asia. To be able to perform ENB under IVS will be of great advantage in view of the high volume of cases expected in Asia, and ENB under IVS allows greater access of this technology to patients and practitioners.
This article describes the techniques involved in biopsying small PNs by ENB under IVS in the authors’ department. The techniques aim to maximise the yield of ENB and related techniques under IVS.
ENB under IVS
Sedation
Intravenous opioid and benzodiazepine and/or propofol are necessary for ENB performed under IVS. Opioids that have been used by various authors include fentanyl and morphine; midazolam is the preferred benzodiazepine (12). It should be noted that ENB tends to be a more prolonged procedure when compared to conventional FB (mean 8–9.3 additional minutes) (13,14), with a total procedure time variously reported between 25.7 and 70 minutes (6,7). Therefore it is often necessary to give additional boluses of IVS during the procedure if the effect of the previous doses is wearing off, with higher total doses. Well-trained physicians, nurses and/or respiratory therapists proficient in airway management during moderate to deep IVS are highly recommended to manage the patient during ENB (8). The authors’ department prefers IV pethidine/diazepam for their longer half-lives than fentanyl/midazolam combination.
Topical anaesthesia
Lidocaine gel (2%) can be used for nasal topical anaesthesia. Lidocaine spray (10%, 10 mg per actuation, 2–5 actuations) provides oro-pharyngeal topical anaesthesia. Lidocaine (1%, 10 mg/mL) should be used to provide topical anaesthesia of the vocal cords and trachea-bronchial tree during ENB, using a spray-as-you-go technique as in standard FB (15). Similar to IVS, it is often necessary to give additional aliquots of lidocaine during ENB to maintain topical anaesthesia of the vocal cords and trachea-bronchial tree.
Current evidence suggests that lidocaine doses up to 15.4 mg/kg may be used without serious adverse events, but subjective symptoms of lidocaine toxicity (e.g., dizziness, euphoria) were reported in studies using ≥9.6 mg/kg lidocaine (15). It is the authors’ practice to limit total lidocaine dose (nasal, oropharyngeal, vocal cords and trachea-bronchial tree) to <9.6 mg/kg especially in patients with cardiac, hepatic or renal dysfunction, and in older patients.
Anti-cholinergics (e.g., atropine, glycopyrrolate) are not routinely recommended during FB (15). In an occasional patient in whom excessive secretions interfere with the alignment process (between CT images and patient’s anatomy) for ENB, or obscure the visualization of small peripheral bronchi, anti-cholinergics may become necessary.
Monitoring
All patients undergoing ENB should have heart rate (continuous electrocardiogram), respiratory rate, blood pressure and oxygen saturation monitored regularly before, during and after the procedure as for standard FB (15).
ENB
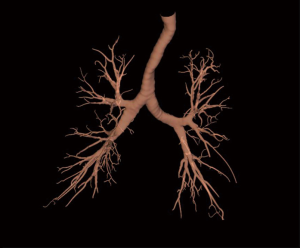
Excellent review is available that describes the techniques of ENB performance in details (12). Briefly, during the planning phase, data from CT images is loaded onto an ENB software. The target lesion is identified, and pathways to the lesion (via the bronchial tree) are planned semi-automatically with the planning software. Once planning is complete, a 3-dimensional reconstructed view of the bronchial tree (Figure 1) with planned route(s) to the lesion is generated by the ENB software (Figure 2A,B,C,D). Animated VB that replicates the exact view that will be seen during ENB is also available with the software (Figure 3A,B).
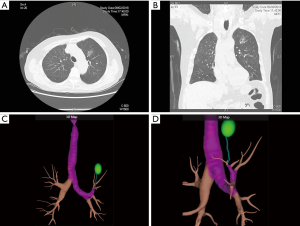
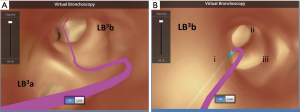
During the procedure phase, a surveillance bronchoscopy is first performed to clear secretions, instill topical anaesthetic and exclude endobronchial lesion. A location board that emits electromagnetic waves is placed on the patient’s body. A sensing device is then inserted into the working channel of the bronchoscope, and the bronchoscope is advanced into various part of the bronchial tree to allow automatic matching of the CT images with the real life anatomy of the patient (registration). Once registration is satisfactory, navigation can proceed. The bronchoscope is guided by the sensing device to reach the lesion (analogous to driving with Global Position System guidance), using pre-planned pathway(s) within VB images that are displayed side-by-side with the actual bronchoscopic images. When the tip of the sensing device is aligned with and in close proximity to the target lesion, it is recommended to confirm navigation success with R-EBUS before sampling, which will increase diagnostic yield (4).
Sampling methods
It is important to recognize that navigation success does not necessarily equate sampling success. Various anatomical and technical factors may negatively impact sampling success in ENB. One example is the small calibre of bronchus leading to the small PN, which may restrict the opening of the biopsy forceps. Another example is a lesion that does not have a “bronchus sign” on CT. This feature is shown to be associated with a lower yield in meta-analysis (6). Rapid on site evaluation (ROSE) may help to assess the quality of the sampled materials to make a diagnosis, and may help to decide whether additional samples are necessary (6,7).
Sampling methods of ENB is similar to conventional FB, which includes transbronchial needle aspiration (TBNA), TBBx, brush cytology, targeted bronchoalveolar lavage (BAL). Catheter suction (aspirating material by continued suction by a 10-mL syringe and moving the catheter back and forth) was reported to increase the diagnostic yield over TBBx (16). Biopsy forceps and cytology brush generally follows that path of the airway, whereas transbronchial needle and needle brush can penetrate outside the bronchial wall (“off-road” tools). The authors generally use TBNA and needle brush if the lesion does not have a bronchus sign, or is not directly aligned with the supplying bronchus, in addition to TBBx and regular brush cytology.
Complications of ENB
Severe complications are seldom encountered. A pneumothorax rate of 3.1% (95% CI: 2.1–4.3%) was reported, in which 1.6% (95% CI: 1.0–2.6%) required chest tube drainage. Minor or moderate bleeding was reported in 0.9% (95% CI: 0.4–1.6%), none of them required specific treatment (6). It is believed that the bronchoscopic instrument (e.g., extended working channel) that is wedged in the sub-segment harbouring the PN provides tamponade of the bleeding source.
Manual mapping
Because of the relatively long procedure time of ENB when compared to conventional FB, it is desirable to contain or reduce the procedure time of the former, so that patients are not given IVS and topical anaesthesia excessively. The authors find it rewarding to spend more time at the planning phase, and to mentally rehearse the pathways to the lesion before the actual navigation. This will allow the navigation to be more focused at the likely bronchial branch(es) leading to the lesion at each successive generation, instead of a trial-and-error approach to test each potential division.
To assist the mental rehearsal, it is useful to manually draw map of successive bronchial branches leading to the lesion. Manual mapping method has previously been described using thin-cut CT thorax with detailed analysis of the bronchial branches (17). The ENB software provides a 3-dimensional reconstructed view of the bronchial tree and animated VB with the planned route to the target lesion. Such features facilitate manually drawing of bronchial map to the lesion (Figure 4). Understanding the map of the bronchial branch(es) leading to the lesion allows rapid navigation to the target.
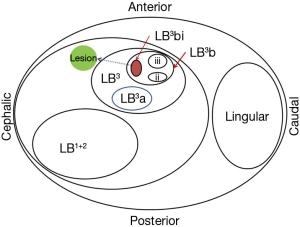
For the purpose of accurate documentation, it is useful to name successive bronchial branches systematically. The authors modify from a previously described nomenclature system (18) as follows:
- The segmental bronchus is designated according to the Nomina Anatomica (e.g., RB1, RB2, etc.);
- The more peripheral bronchi are designated sequentially, using (a/b/c, i/ii/iii, α/β/γ, x/y/z) for third-, fourth-, fifth- and sixth-order bronchi;
- The axial plane is considered first, with the more apical bronchus receiving the smaller letter/number;
- The antero-posterior axis is considered the second, with the more posterior bronchus receiving the smaller letter/number;
- Then the lateral bronchus receives the smaller letter/number.
An example is given in Figure 4.
Alternatives to ENB
There will be a minority of patients who cannot be adequately sedated with the above approach, making ENB (registration and/or navigation, see below) difficult. In such circumstance, conversion to examination by an ultra-thin bronchoscope (UTB) with multi-modality guidance (vide infra) usually allows the procedure to be continued with better tolerance because of a smaller bronchoscope size. If ENB is not possible and UTB is non-diagnostic, patient may need to have ENB under GA or have surgical biopsy of the PN.
Ultra-thin bronchoscopy guided by multi-modal devices
Apart from ENB, TBBx using R-EBUS and VB have been reported to have good diagnostic yield for small PNs (19,20). Since the ENB software provides 3-dimensional images of the bronchial tree and animated VB, it can be easily adapted to guide an UTB to the target. Using the manual mapping method with VB images described above, UTB assisted by R-RBUS and fluoroscopy (21) can be used to target PNs without the need for electromagnetic guidance, with potential for substantial cost savings.
There is no randomized controlled trial directly comparing the diagnostic yields of ENB with UTB/R-RBUS/VB. In the interim, the authors favour the use ENB in patients with:
- unclear distal bronchial branches (due to imperfect CT quality), or
- ground glass lesions (less distinct on R-RBUS when compared to solid lesion), or
- lesion adjacent to a bronchial branch, rather than surrounding a bronchial branch, in which the diagnostic yield was reported to be less with R-EBUS (22).
On the other hand, patient’s tolerance to UTB maybe better in view of the smaller size (3.0 mm). The authors routinely prepare an UTB to back up ENB in case the patient does not tolerate ENB.
Conclusions
ENB is gaining acceptance and popularity amongst bronchoscopists as one of the tools to diagnose PNs. To be able to perform it under IVS may improve access of the technology to practitioners and patients, which is an advantage in Asia in view of the high PN burden. VB software and manual mapping can facilitate navigation, and potentially shorten procedure time. They also facilitate the use of UTB (in combination with R-RBUS and fluoroscopy) as an alternative to ENB in specific patient subgroups.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer. ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-48S.
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomised controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Leong S, Shaipanich T, Lam S, et al. Diagnostic bronchoscopy – current and future perspectives. J Thorac Dis 2013;5:S498-510. [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: A systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Zhang W, Chen S, Dong X, et al. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis 2015;7:799-809. [PubMed]
- Bowling MR, Kohan MW, Walker P, et al. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J Bronchology Interv Pulmonol 2015;22:5-13. [Crossref] [PubMed]
- Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-ii54. [Crossref] [PubMed]
- Phua CK, Sim WY, Tee KS, et al. Evalution of pulmonary nodules in Asian population. J Thorac Dis 2016;8:950-7. [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of pulmonary nodules. Clinical practice consensus guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: a descriptive analysis. J Thorac Dis 2012;4:173-85. [PubMed]
- Hautmann H, Schneider A, Pinkau T, et al. Electromagnetic catheter navigation during bronchoscopy: validation of a novel method by conventional fluoroscopy. Chest 2005;128:382-7. [Crossref] [PubMed]
- Becker HD, Herth F, Ernst A, et al. Bronchoscopic biopsy of peripheral lung lesions under electromagnetic guidance: a pilot study. J Bronchology Interv Pulmonol 2005;12:9-13.
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Eberhardt R, Morgan RK, Ernst A, et al. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigation bronchoscopy. Respiration 2010;79:54-60. [Crossref] [PubMed]
- Zhang L, Tong R, Wang J, et al. Improvements to bronchoscopic brushing with a manual mapping method: a three-year experience of 1143 cases. Thoracic Cancer 2016;7:72-9. [Crossref] [PubMed]
- Jafek BW, Carter DR. Endoscopic nomenclature for bronchopulmonary anatomy. Otolaryngol Head Neck Surg 1979;87:815-7. [Crossref] [PubMed]
- Asahina H, Yamazaki K, Onodera Y, et al. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation. Chest 2005;128:1761-5. [Crossref] [PubMed]
- Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430-40. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions. Am J Respir Crit Care Med 2015;192:468-76. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]


