Endobronchial ultrasound-guided transbronchial needle aspiration and cervical mediastinoscopy for mediastinal staging of non-small cell lung cancer: a retrospective comparison study
Introduction
Accurate mediastinal lymph nodes staging is essential for non-small cell lung cancer (NSCLC) patients who are potential candidates for radical surgical resection. Mediastinal staging is the most important factor that affects patient’s treatment strategy and prognosis (1). Common staging methods include non-invasive and invasive staging. Non-invasive methods are imaging-based, such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and PET-CT scans, which had poor sensitivity and specificity (2). For patients with potential resectable NSCLC, invasive methods are indispensable for mediastinal lymph nodes staging (N-staging) (3-5).
Transbronchial needle aspiration (TBNA) guided by endobronchial ultrasound (EBUS) was first introduced to clinical practice in 2002, and soon, physicians had found that linear probe EBUS-guided TBNA increased the yield of mediastinal lymph nodes diagnosis (6,7), which changed the practice of bronchoscopic biopsy of the mediastinum. Generally, TBNA had a sensitivity of around 50–60% in invasive staging(3). Guided by the linear probe of real-time EBUS, the sensitivity can be improved up to 85% (6,7). Recently research had indicated that the yield of EBUS-TBNA for mediastinal lymph node staging in lung cancer had increased to 90% or higher (8-16).
In past decades, mediastinoscopy had generally been considered a favorable option for invasive mediastinal staging (17,18). EBUS-TBNA had been developing rapidly and recommended for NSCLC mediastinal staging by clinical practice guidelines (19,20). Derived from an imaging modality that is capable of detecting lymph nodes using a probe, EBUS-TBNA has satisfactory sensitivity and specificity in pathological results by invasive needle aspiration and fewer injuries compared to mediastinoscopy (8-14).
Currently, minimally invasive needle techniques, like EBUS-TBNA, have been increasingly accepted as the first choice for mediastinal disease diagnosis. However, only few comparison studies were focused on mediastinal lymph nodes staging of NSCLC by EBUS-TBNA and cervical mediastinoscopy (CMS) methods (7,14). So, we conducted this retrospective study to compare the diagnostic yield of malignant mediastinal lymph nodes and N staging of EBUS-TBNA and CMS in clinical suspected lung cancer with enlarged mediastinal lymph nodes.
Methods
A total of 248 patients underwent EBUS-TBNA and 303 patients underwent CMS between January 2009 and March 2016 in the Sun Yat-sen University Cancer Center. The inclusion criterion of the final analysis set included clinical suspected lung cancer, which was based on symptoms, smoking history, or other characteristics; with enlarged mediastinal lymph nodes (short axis ≥10 mm) based on enhanced CT; patients should not receive any anti-cancer treatment. Clinical/radiologic evidence of stage IV or N3 lung cancer, or other mediastinal masses (e.g., thymoma, lymphoma) before the EBUS-TBNA or CMS were excluded. Patients with pathological confirmed NSCLC, and underwent substantial surgical resection with systematic mediastinal lymphadenectomy (SML) were included in the analysis set. The sizes of all lymph nodes (max axis and minor axis) were measured and recorded according to enhanced CT scan images. To be qualified in per lymph node station diagnosis yield comparison, the enlarged mediastinal lymph nodes were confined in station #2, #4 and #7 in both EBUS-TBNA and CMS groups. Radical resection and SML were considered as the gold standard in this study. Consecutive patients were included into this retrospective study in single center. Invasive mediastinal staging methods (whether EBUS-TBNA or CMS) were decided mainly by surgeon according to patients’ clinical characteristics (age, lesions on CT scan, complication diseases, performance status, etc.). Both EBUS-TBNA and CMS were conducted by specialized physicians, and an independent review board of Sun Yat-sen University Cancer Center approved the data collection and analysis (Approved number: B2017-101-01).
EBUS-TBNA
EBUS-TBNA was performed as a separate procedure before radical resection and SML. After airway examination with conventional bronchoscopy, EBUS-TBNA was performed under conscious sedation. An ultrasound probe (BF-UC260F-OL8; Olympus) was inserted into the trachea with a flexible bronchoscope. TBNA biopsies were performed using a dedicated 22-gauge needle (NA-201SX-4022, Olympus) (21). After the procedure, rapid on-site evaluation (ROSE) was conducted for cytological smears using the collected samples at first, the remain samples would be used for Thinprep cytologic test, histological examination would be performed using the fresh tissue sample, immunohistochemistry was necessary in some cases. Quality control: sample adequacy was decided by endoscopy physician and pathologist, the puncture was performed at most 4 times. Large quantity of lymphocytes would be considered as representative. NSCLC lymph node metastasis, highly suspicious cancer cell would be diagnosed positive. Negative results should be no malignant cells found in cytological or histological examination. Non-diagnostic sample included blood clots, necrotic tissue, mucus, etc. (Figure 1).
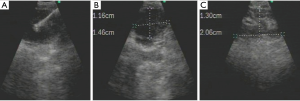
CMS
CMS conducted in this analysis was mostly performed as a part of lung cancer radical resection. Whether substantial surgeries continue or not depended on frozen pathology analysis and the surgeons' decisions. If the frozen pathology analysis indicated N2 or N3 nodes positive, surgery would generally be ceased, and patients received neoadjuvant therapy or other treatments, unless the surgeon had other indications to continue. If N1 nodes positive (in EBUS-TBNA group) or all nodes negative, the surgeon would generally continue with the radical resection and SML in resectable cases or cease surgery in medical inoperable cases.
According to the regional lymph node staging of lung cancer by the IASLC 2009 criteria, EBUS-TBNA was able to reach N2 nodes including superior mediastinal nodes (stations #1 highest mediastinal, #2 upper paratracheal, #3 retrotracheal and #4 lower paratracheal), inferior mediastinal nodes (stations #7 subcarinal), and N1 nodes including hilar (#10), interlobar (#11), and part of lobar (#12). EBUS-TBNA cannot reach #3 pre-vascular, #5 sub-aortic, #6 para-aortic, #8 paraesophageal, #9 pulmonary ligament, and #13 segmental, #14 subsegmental lymph nodes. The lymph nodes of CMS are fewer, it only includes N2 nodes #1 highest mediastinal, #2 upper paratracheal, #4 lower paratracheal and #7 subcarinal (19,22-24).
Statistical analyses
Statistical analyses were performed using SPSS v13 statistical software (USA). Continuous variables were expressed as means and standard deviations, and comparisons were performed with t tests. Categorical variables are summarized as count and percent. Pearson Chi-square test or Fisher’s exact test, as appropriate, was used for comparing proportions. McNemar’s test was used for evaluating agreement between the two procedures. A two-tailed p value of 0.05 indicated statistical significance.
Results
The study flowchart is shown in Figure 2. In total, 103 and 274 clinical suspected lung cancer patients with indication for mediastinal lymph nodes staging received EBUS-TBNA or CMS. Two hundred and forty-five patients met the inclusion criterions and enrolled into analysis set, 55 patients in EBUS-TBNA group and 190 patients in CMS group, respectively. Seven patients (6.8%) in EBUS-TBNA group were excluded for non-diagnostic sample, standard cytological examinations were conducted in the biopsied lymph nodes in these patients, 5 were diagnosed as necrotic tissue, 2 were blood clots. Two patients underwent both EBUS-TBNA and CMS, and were included in both groups.
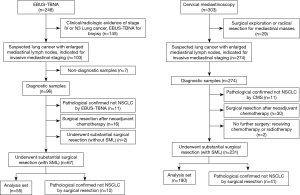
Patient demographics were shown in Table 1, basic characteristics were balanced in two groups. Three hundred and six mediastinal lymph nodes met the criterion and enrolled into the comparison, 62 and 244 nodes were biopsied by EBUS-TBNA and CMS respectively. The average number of nodes per case were 1.13 and 1.28, respectively.

Full table
Mediastinal lymph nodes diagnosis and N staging yield comparison
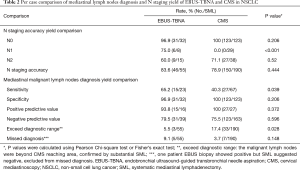
The results were showed in Table 2. Fifty-five patients underwent surgical resection and SML after EBUS-TBNA examination. Forty-six patients had consistent pathological confirmed N staging results from both TBNA and surgery. Nine patients showed a disagreement of N stage with SML, and three patients had positive lymph nodes exceed diagnostic range of EBUS-TBNA (1 node in #5, and 2 nodes in #13). One patient had #4 positive but SML indicated a negative result. Five patients were missed diagnosed, EBUS suggested negative but SML indicated positive (1 nodes in #2, #4 and #7 each) in 3 patients, 2 patients were missed because of small nodes (1 node in #4 and #7 each, short-axis <10 mm). One hundred and ninety patients underwent radical surgery substantially after CMS, 150 patients had consistent results, 33 patients with positive lymph nodes metastasis exceed CMS’s diagnostic range (1 node in #5; 2 nodes in #6, #8 and #9 respecively;19 in #10, 13 in #11, 6 in #12, 20 in #13), and 7 patients were with missed diagnosis, CMS suggested negative but SML indicated positive (2 nodes in #7 and 1 in #4) in 3 patients,4 patients were missed for small nodes (1 node in #2 and #7 each, 2 nodes in #4, short-axis <10 mm).
Full table
The accuracy rate for N staging in EBUS-TBNA was 83.6% (46/55 cases) versus 78.9% (150/190 cases) in CMS (P=0.444), with no statistically significant differences. EBUS-TBNA had significant higher sensitivity (65.2% vs. 40.3%, P=0.039) in mediastinal malignant lymph nodes diagnosis, with also higher missed diagnosis rate (9.1% vs. 3.7%, P=0.148) than CMS. EBUS-TBNA also had a wider diagnostic range, the exceeding rate was significantly lower than CMS (5.5% vs. 17.4%, P=0.028).
Comparison of lymph nodes station diagnosis yield
Lymph nodes station #2, #4 and #7 were in the diagnostic range of both EBUS-TBNA and CMS, which made it comparable to evaluate the diagnosis yield of enlarged lymph nodes (short-axis ≥10 mm in CT scan). The results were showed in Table 3, both EBUS-TBNA and CMS had very high levels and no statistically difference was found in diagnosis sensitivity and specificity (82.4% vs. 94.7%, P=0.130; 97.4% vs. 100%, P=0.173; respectively) of these 3 mediastinal lymph nodes station. The diagnostic accuracy was also at very high level; however, CMS was slightly better than EBUS-TBNA (98.8% vs. 92.9%, P=0.025).

Full table
The description of lung cancer lymph nodes
The sizes of all the lymph nodes dissected in SML were measured according to previous CT scan and described in Table 4. Short-axis of malignant lymph nodes were significantly longer than benign lymph nodes (mean 14.2 vs. 6.5 mm, P<0.001). The results also indicated that for the detection of malignant lymph nodes with short axis ≥15 mm, CMS had better diagnostic yield than EBUS-TBNA (100% vs. 80%, P=0.012). Malignant rate of lymph nodes was elevated in accordance with the minor axis, and reached as high as 48.8% when minor axis ≥15 mm.

Full table
Safety
There were 4 complications (1.6%, 4/248) that occurred in EBUS-TBNA examination. Two patients had a severe cough, and two patients experienced an oxygen saturation decrease during the process and were not able to complete the TBNA. Seven complications (2.3%, 7/303) were observed in CMS. Six patients had recurrent laryngeal nerve or vessel injury, and one patient suffered from a post-operative infection. Generally, the complication rate was low in both invasive examinations. When CMS was conducted as a part of surgical resection, complications were difficult to assess.
Discussion
Over the past decade, interest had been drawn towards exploring the roles of EBUS-TBNA in lung cancer mediastinal lymph node staging, not only by ultrasound specialists, but also by thoracic surgeons. A prospective, crossover trial to compare the diagnostic yield of EBUS-TBNA and CMS was conducted by Ernst in 2008, and the results indicated that the yield of N staging accuracy was not different between two methods, but the sensitivity of EBUS-TBNA in lymph node station diagnosis was higher than that of CMS (25). Several systemic reviews also concluded that the pooled sensitivities of EBUS-TBNA and CMS had no significant differences, and both exhibited equally high diagnostic accuracy for mediastinal staging of lung cancer (20,26).
In our study, we retrospectively analyzed suspected lung cancer patients with enlarged mediastinal lymph nodes who underwent EBUS-TBNA or CMS for invasive mediastinal staging, using SML as the reference standard. Our results also indicated that both EBUS-TBNA and CMS had similar diagnostic accuracy in N staging (83.6% and 78.9%). However, EBUS-TBNA had better diagnostic sensitivity than CMS in mediastinal malignant lymph nodes. Most of the disagreement in N staging of CMS group were malignant lymph nodes beyond the diagnostic range, mostly happened in N1 station; for those within the range, the missed diagnosed rate was very low (3). The disagreement of EBUS-TBNA in N staging was more balanced. So, one advantage for EBUS-TBNA in NSCLC mediastinal lymph nodes diagnosis and N staging is the wider diagnostic range, which allows the physician to explore both N2 and N1 (such as hilar, interlobar, and lobar) lymph nodes station. Combined EBUS with esophageal ultrasound (EUS), the diagnostic range could reach the aortopulmonary (#5), paraesophageal (#8) and inferior pulmonary ligament (#9) nodes to accomplish complete endoscopic staging of the N2 mediastinal lymph nodes staging (5,27).
However, in EBUS-TBNA, the pathology sample collected by lymph nodes needle aspiration is less excessive, mostly for cytology only. CMS is performed mainly by lymph nodes resection, provide both cytology and histology samples, which can significantly decrease the missed diagnostic rate (3). The higher missed diagnosis rate could be seen in lymph nodes station #2, #4 and #7 diagnosis yield comparison, the diagnostic accuracy was higher in CMS group. Our results remain consistent with prior reports’ conclusions (3,10-16,25). Also, CMS has significant higher diagnostic yield in lymph nodes ≥15 mm in our study. The high false negative rate in EBUS-TBNA could largely attribute to inadequate sample. But, the results are less convincible because of small cohort size, large-scale sample studies are needed to confirm that results. CMS operation requires inpatient care and general anesthesia, and is associated with complications such as nerve and vessel damage. EBUS-TBNA has fewer complications and less damage to patients, and could be operated in a clinic with topical anesthesia (28-30).
Our study suggests that EBUS-TBNA might be preferred in diagnosis of enlarged lymph nodes in suspected lung cancer, however, there are still concerns about EBUS-TBNA, and CMS clearly retains an important role. One of the concerns is the false negative rate of EBUS-TBNA, previous report has suggested a false negative rate as high as 24% (nearly 10% in our study), which is commented that needle-based biopsy is not as reliable as surgical resection for less abundant sampling (3,17). Another concern is the high non-diagnostic rate of EBUS-TBNA, which is reported as high as 25.8% (6.7% in our study, 7/105), could arouse a diagnostic bias and clinical confusions.
So, the appropriate practice, which is also suggested by the guideline, is EBUS-TBNA and followed by CMS. EBUS-TBNA can be easily repeated without the technical difficulties, combination examination is reasonable (20,31). Clinical suspected NSCLC will be mediastinal staged by radiology at first, EBUS-TBNA will be performed to confirm the malignant nodal involvement, and negative results should be corroborated by CMS. Based on these results, physician could make decisions of substantial radical resection or neoadjuvant chemotherapy or other treatments.
Limitation
We recognize various limitations of the present study. This is a retrospective cohort study, perspective randomization is not available. So, selection bias (EBUS-TBNA or CMS) does exist and may affect the results. Also, there is inherent investigator bias in deciding went on or cease surgery after frozen pathology results of CMS, so the surgery rate in CMS group is higher than EBUS-TBNA group. Interpersonal bias might be small as the basic characteristics are balanced in both groups.
Conclusions
The results of our study suggest that the diagnostic accuracy for EBUS-TBNA and CMS are similar, but EBUS-TBNA had better malignant diagnostic sensitivity and fewer complications, which indicates that in clinical suspected lung cancer patients with enlarged mediastinal lymph nodes, EBUS-TBNA is preferred for invasive mediastinal nodal staging.
Acknowledgements
Funding: This study was funded by National Key R&D Program of China (grant No. 2016YFC0905500), The Chinese National Natural Science Foundation project (grant No. 81372502, No. 81602011 and No. 81773244), Wu Jieping Medical Foundation Project (grant No. 3206740.10012).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the independent review board of Sun Yat-sen University Cancer Center (approved number: B2017-101-01) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consents were exempted in this retrospective study.
References
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S.
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-220S.
- Medford AR, Bennett JA, Free CM, et al. Mediastinal staging procedures in lung cancer: EBUS TBNA and mediastinoscopy. Curr Opin Pulm Med 2009;15:334-42. [Crossref] [PubMed]
- Yasufuku K, Fujisawa T. Staging and diagnosis of non-small lung cancer: Invasive modalities. Respirology 2007;12:173-83. [Crossref] [PubMed]
- Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003;123:604-7. [Crossref] [PubMed]
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Bauwens O, Dusart M, Pierard P, et al. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer 2008;61:356-61. [Crossref] [PubMed]
- Hwangbo B, Kim SK, Lee HS, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009;135:1280-7. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Zhang R, Mietchen C, Krüger M, et al. Endobronchial ultrasound guided fine needle aspiration versus transcervical mediastinoscopy in nodal staging of non-small cell lung cancer: a prospective comparison study. J Cardiothorac Surg 2012;7:51. [Crossref] [PubMed]
- Vincent BD, El-Bayoumi E, Hoffman B, et al. Real-time endobronchial ultrasound guided transbronchial lymph node aspiration. Ann Thorac Surg 2008;85:224-30. [Crossref] [PubMed]
- Herth FJ, Rabe KF, Gasparini S, et al. Transbronchial and transoesophageal (ultrasound-guided) needle aspirations for the analysis of mediastinal lesions. Eur Respir J 2006;28:1264-75. [Crossref] [PubMed]
- Shrager JB. Mediastinoscopy: still the gold standard. Ann Thorac Surg 2010;89:S2084-9. [Crossref] [PubMed]
- Margaritora S, Cesario A, Galetta D, et al. Mediastinoscopy as a standardized procedure for mediastinal lymph node staging in non-small cell carcinoma, Do we have to accept the compromise? Eur J Cardiothorac Surg 2001;20:652-4. [Crossref] [PubMed]
- Yasufuku K, Chhajed PN, Sekine Y, et al. Endobronchial ultrasound using a new convex probe: a preliminary study on surgically resected specimens. Oncol Rep 2004;11:293-6. [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Luo GY, Cai PQ, He JH, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration in the management of mediastinal and hilar lymphadenopathy without intrapulmonary mass: experience from the largest cancer center of southern China. Cell Biochem Biophys 2013;67:1533-8. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Walles T, Friedel G, Stegherr T, et al. Learning mediastinoscopy: the need for education, experience and modern techniques-interdependency of the applied technique and surgeon’s training level. Interact Cardiovasc Thorac Surg 2013;16:450-4. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Ge X, Guan W, Han F, et al. Comparison of Endobronchial Ultrasound-Guided Fine Needle Aspiration and Video-Assisted Mediastinoscopy for Mediastinal Staging of Lung Cancer. Lung 2015;193:757-66. [Crossref] [PubMed]
- Herth FJ, Lunn W, Eberhardt R, et al. Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med 2005;171:1164-7. [Crossref] [PubMed]
- Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999;118:894-9. [Crossref] [PubMed]
- Porte H, Roumilhac D, Eraldi L, et al. Therole of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg 1998;13:196-9. [Crossref] [PubMed]
- Vueghs PJ, Schurink GA, Vaes L, et al. Anesthesia in repeat mediastinoscopy: a retrospective study of 101 patients. J Cardiothorac Vasc Anesth 1992;6:193-5. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]


