Novel risk factors for the healthcare associated infections (HAIs) in patients with Stanford type A aortic dissection (TAAD)
Introduction
Thoracic aortic dissection (TAD) occurs when blood flow is redirected from the aorta (true lumen) into the media of the aortic wall (false lumen), through an intimal laceration, creating a septum (1). Aortic dissection (AD) is a catastrophic disease process, with an age-dependent incidence ranging from between 3.5 and 6/100,000 person-years in the general population to as high as 10/100,000 person-years in the elderly (2,3). The Stanford system is applied widely to classify AD and surgical treatment is the primary choice for type A aortic dissection (TAAD) (4). Delay in diagnosis and surgical treatment is associated with increased mortality (5). Presentation with TAAD is associated with high mortality and a 5-year survival of 32%, with operative therapy indicated to improve survival (2,6,7). Besides, it is worth noting that TAAD underwent surgical procedures always included some clinical features which would were dramatically associated with elevated risks of HAIs, such as long duration of surgery, the application of mechanical ventilator, blood transfusion (8), femoral vein catheterization (9,10). Moreover, HAIs are deemed the most frequent adverse event threatening patients’ safety worldwide (11-13) which considerably increase the burden of in-patients, such as hospital acquired pneumonia (HAP), ventilator associated pneumonia (VAP), bloodstream infections (BSIs), surgical site infections (SSIs), gastrointestinal tract infection (GI). However, there are few studies on the risk factors and HAIs for the patients with TAAD after ascending aortic and arch replacement under deep hypothermic circulatory arrest (DHCA) during hospitalization. In this study, we aimed to identify the risk factors of HAIs for the patients following surgical repair of TAAD and optimized infection prevention.
Methods
Patient selection
We retrospectively reviewed the medical records of all the patients diagnosed with TAAD admitted to The First Affiliated Hospital of Nanjing Medical University between Jan 01, 2013 to May 31, 2016. All the patients with TAAD was defined according to the 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease (14) and diagnosed by computed tomography arteriography (CTA). All the patients with TAAD were first diagnosed and surgical treatment in our facility. The definition of hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) is described in ATS/DSA 2016 guideline (15). We applied CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections (16). This study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (ID: IRB-SOP-AF17).
Data collection
All the authors discussed and approved the standard form. Relative clinical variables were collected and recorded on a standard form by two author independently (Chen WS and Ni BQ) that included information on patient age, gender, underlying disease (hypertension, Marfan syndrome, tumor, liver disease, kidney disease), surgical parameters (operation time, DHCA time, myocardial ischemia time, brain per-fusion time), laboratory examination [serum D-dimer, white blood cell (WBC), neutrophil, lymphocyte, platelet (PLT)], duration of hospital stay, duration of intensive care unit (ICU) stay, and type of HAIs. All data come from the patients who first admitted to our hospital and treated underwent ascending aortic and arch replacement under DHCA.
Statistical analysis
Primary analysis compared patients with TAAD with and without HAIs. Categorical variables were compared using χ2 analysis or Fisher exact test where appropriate. Continuous variables were analyzed with 2-sample t-tests or Wilcoxon rank-sum tests.
A stepwise multiple logistic regression model was applied to test the association analysis of risk factors for the HAIs in patients with TAAD after surgical treatment. In the logistic regression model, each regression coefficient is the logarithm of the odds ratio (OR). A Hosmer-Lemeshow test were used for evaluation the goodness-of-fit and the final model was built by forward selection procedure in which only the variables reaching the conventional significance level of 0.05. We investigated also the predictive capability of the logistic model by means of the area under the receiver operating characteristic (ROC) curve (AUC) (17). In this study, we suggest that values between 0.6 and 0.7 be considered as indicating a weak predictive capacity, values between 0.71 and 0.8 a satisfactory predictive capacity and values greater than 0.8 a good predictive capacity (18). Data were double entered serially using patients’ codes and were only analyzed at the end of the study. All statistical analyses were performed with Stata version 11.2 by adopting a significance level of alpha =0.05.
Results
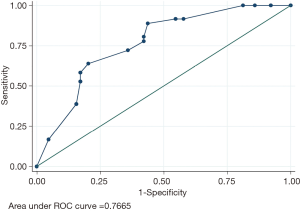
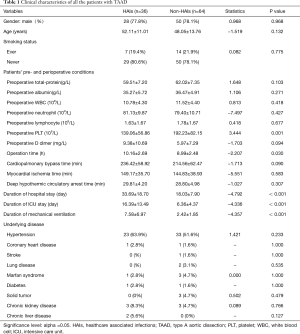
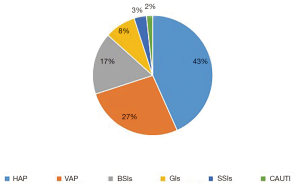
In all, 210 patients with AD admitted to our hospital, 100 patients had TAAD (100/210, 47.62%), generating 36 HAIs. Therefore, all the TAAD patients were then allocated to the HAIs group (n=36) and Non-HAIs group (n=64). We found that age, gender and underlying diseases were similar between the HAIs and Non-HAIs groups (all P values >0.05) (Table 1), indicating that two groups balanced. Among the 36 TAAD patients with HAIs, there are 60 episodes HAIs, including 26 HAP (43%), 16 VAP (27%), BSIs (17%), GIs (8%), 2 organ/space SSIs (3%) (sternal infection after surgery), 1 CAUTI (2%) (Figure 1). Meanwhile, univariate analysis showed that preoperative WBC, preoperative neutrophil, preoperative lymphocyte, preoperative D-dimer, cardiopulmonary bypass time, myocardial ischemia time, DHCA time were not significant between HAIs group and Non-HAIs group (all P values >0.05; Table 1). Preoperative PLT, operation time, duration of hospital stay, duration of ICU stay, were both significantly higher in HAIs group than in the Non-HAI group (139.06±56.86 vs. 192.23±82.15, P=0.001; 10.16±2.69 vs. 8.99±2.48, P=0.030; 33.69±18.70 vs. 18.03±7.90, P<0.001; 16.39±13.49 vs. 6.36±4.37, P<0.001 respectively) (Table 1). Forward stepwise was applied to build the final multivariable model. We found that [DCHA >29 min; OR =2.60; 95% confidential interval (CI), 1.01–6.80; P=0.048], preoperative PLT <171×109/L (OR =3.62; 95% CI, 1.33–9.79; P=0.011) and D-dimer >4.25 mg/L (OR =2.83; 95% CI, 1.07–7.47; P=0.035) were independently associated with the increased risks of HAIs for the patients with TAAD following surgical repair (Table 2). Hosmer-Lemeshow statistic of the model suggested perfect model discrimination from a perfect fit (χ2=4.77, P=0.6883). Logistic model was verified when the area under ROC curve was equal to 0.7665 (Figure 2), indicating a satisfactory predictive capacity.
Full table

Full table
Discussion
Patients with TAAD undergoing surgical repair are at higher risk for postoperative infections. Because it usually mixed up a variety of risk factors which contribute to HAIs, such as cardiac surgeries (19), long duration of surgery time, mechanical ventilation. In this study, we firstly investigated the risk factors of HAIs for the patients with TAAD after surgical treatment. We found that the patients with TAAD undergoing arch replacement were prone to a higher HAIs incidence rate (60%, 60 episodes/100 patients), the top three HAIs types were HAP (43%), VAP (27%) and BSIs (17%). The inflammation after TAAD, long duration of DHCA, and inadequate cooling of the lungs during surgery (20), intraoperative transfusion of banked blood (21) would increase the risk of pneumonia. Multivariate logistic regression analysis indicated that longer DHCA was an independent risk factor for postoperative HAIs. DHCA during surgical treatment of TAAD patients induces cytokine-mediated inflammation during CPB, and leukocyte infiltration into the pulmonary tissue which would result in postoperative pneumonia (22). In this case, our results suggested that recipients of treatment for TAAD would benefit from reductions in the DHCA times with improved surgical techniques. Meanwhile, fragments of banked blood deposited in the lung interstitial activated inflammation elevated lung damage, which may result in pneumonia. All of these causes may contribute to the most common postoperative HAP and VAP.
When AD occurs, blood emitted from the ventricle influx into the false lumen, and the circulation of PLTs content for the protection effect of the break. PLT activation and acute AD are associated with aortic wall tearing range (23,24). Our study found that lower PLTs in concentration were significantly elevated risk factor for postoperative HAIs (OR=3.62; 95% CI, 1.33–9.79; P=0.011).
D-dimer is a typical degradation product of cross-linked fibrin (25), whose serum level is applied to diagnose deep venous thrombosis (DVT), pulmonary embolism, diffuse intravascular coagulation (DIC). Elevation of the serum D-dimer level reflects the fibrinolytic activity in responses to thrombosis of the false lumen and activation of the extrinsic pathway of the coagulation cascade with an injured aorta (26,27). Among the patients with TAAD, a highly elevated level of serum D-dimer also hints the anatomical extent of the dissection (27). The extent of the dissection is closely related to the difficulty of surgery, operation time, blood transfusion, all of which are important risk factors for HAIs after surgery (25). In our study, we reported that preoperative higher serum D-dimer was significantly associated with increased risk of HAIs.
TAAD patients always endure tissue and organ ischemia and are also likely to cause damage to organ dysfunction, resulting in increased risks of BSI, gastrointestinal tract infection (Figure 1). Therefore, TAAD has a higher incidence of postoperative infections, so we need to establish a simple and rapid diagnostic tool to assess the risk. Our finding suggested that our simple logistic regression model seemed to be a satisfactory tool to predict HAIs, AUC was 0.7665, the diagnostic sensitivity was 63.89%, and the specificity was 79.69%. However, we did not validate the predictive ability in test dataset. Our team will continue to gather more cases and seek external validation to assess the predictive power of the model.
In conclusion, TAAD patients after surgical repair are prone to HAIs. We found that longer DHCA time, lower preoperative PLT and higher preoperative serum D-dimer would significantly increase risks for HAIs, and we should take effective prevention and control measures to guarantee the patients’ safety.
Acknowledgements
Funding: This study was supported by grants from the 2017 Hospital Management Innovation Research Project (grant no. JSYGY-2-2017-205) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD grant no. JX10231801).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (ID: IRB-SOP-AF17).
References
- Kumar A, Kumar K, Zeltser R, et al. Nearly Asymptomatic Eight-Month Thoracic Aortic Dissection. Clin Med Insights Cardiol 2016;10:75-8. [Crossref] [PubMed]
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004;79:176-80. [Crossref] [PubMed]
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Yeh TY, Chen CY, Huang JW, et al. Epidemiology and Medication Utilization Pattern of Aortic Dissection in Taiwan: A Population-Based Study. Medicine (Baltimore) 2015;94:e1522. [Crossref] [PubMed]
- Pacini D, Di Marco L, Fortuna D, et al. Acute aortic dissection: epidemiology and outcomes. Int J Cardiol 2013;167:2806-12. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Teman NR, Peterson MD, Russo MJ, et al. Outcomes of patients presenting with acute type A aortic dissection in the setting of prior cardiac surgery: an analysis from the International Registry of Acute Aortic Dissection. Circulation 2013;128:S180-5. [Crossref] [PubMed]
- Shorr AF, Jackson WL. Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care 2005;11:468-72. [Crossref] [PubMed]
- Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med 2015;373:1220-9. [Crossref] [PubMed]
- Marik PE, Flemmer M, Harrison W. The risk of catheter-related bloodstream infection with femoral venous catheters as compared to subclavian and internal jugular venous catheters: a systematic review of the literature and meta-analysis. Crit Care Med 2012;40:2479-85. [Crossref] [PubMed]
- Burke JP. Infection control - a problem for patient safety. N Engl J Med 2003;348:651-6. [Crossref] [PubMed]
- Bates DW, Larizgoitia I, Prasopa-Plaizier N, et al. Global priorities for patient safety research. BMJ 2009;338:b1775. [Crossref] [PubMed]
- Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011;377:228-41. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-129. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-111. [Crossref] [PubMed]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [Crossref] [PubMed]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36. [Crossref] [PubMed]
- Gasparini G, Bonoldi E, Viale G, et al. Prognostic and predictive value of tumour angiogenesis in ovarian carcinomas. Int J Cancer 1996;69:205-11. [Crossref] [PubMed]
- Zhou ZF, Jia XP, Sun K, et al. Mild volume acute normovolemic hemodilution is associated with lower intraoperative transfusion and postoperative pulmonary infection in patients undergoing cardiac surgery -- a retrospective, propensity matching study. BMC Anesthesiol 2017;17:13. [Crossref] [PubMed]
- Girdauskas E, Kuntze T, Borger MA, et al. Acute respiratory dysfunction after surgery for acute type A aortic dissection. Eur J Cardiothorac Surg 2010;37:691-6. [Crossref] [PubMed]
- Tan TW, Eslami M, Rybin D, et al. Blood transfusion is associated with increased risk of perioperative complications and prolonged hospital duration of stay among patients undergoing amputation. Surgery 2015;158:1609-16. [Crossref] [PubMed]
- Chen MF, Chen LW, Cao H, et al. Analysis of risk factors for and the prognosis of postoperative acute respiratory distress syndrome in patients with Stanford type A aortic dissection. J Thorac Dis 2016;8:2862-71. [Crossref] [PubMed]
- Zhang S, Qian H, Yang Q, et al. Relationship between the extent of dissection and platelet activation in acute aortic dissection. J Cardiothorac Surg 2015;10:162. [Crossref] [PubMed]
- Qin C, Gu J, Qian H, et al. Potential Mechanism of Post-Acute Aortic Dissection Inflammatory Responses: The Role of mtDNA from Activated Platelets. Cardiology 2016;135:228-35. [Crossref] [PubMed]
- Watanabe H, Horita N, Shibata Y, et al. Diagnostic test accuracy of D-dimer for acute aortic syndrome: systematic review and meta-analysis of 22 studies with 5000 subjects. Sci Rep 2016;6:26893. [Crossref] [PubMed]
- Eggebrecht H, Naber CK, Bruch C, et al. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol 2004;44:804-9. [Crossref] [PubMed]
- Weber T, Hogler S, Auer J, et al. D-dimer in acute aortic dissection. Chest 2003;123:1375-8. [Crossref] [PubMed]