Expression and association of CD44v6 with prognosis in T2-3N0M0 esophageal squamous cell carcinoma
Introduction
Esophageal cancer is one of the most common lethal malignancies in the world (1-3), causing more than 400,000 deaths per year (4). In Asian countries, esophageal squamous cell carcinomas (ESCC) accounts for over 90% of esophageal cancers (5). Despite tremendous advances in diagnosis and multimodality therapy, the prognosis of ESCC remains poor and the overall 5-year survival is still less than 15% (1,6). More and more evidence has revealed that classical staging criteria provide insufficient prognostic characterization of ESCC patients, and molecular tumor analysis may provide an effective and feasible means of better defining prognosis (7,8). Therefore, to further improve the survival rate in patients with ESCC, novel prognostic and molecular targets for therapeutic intervention are critically needed for patients with ESCC.
CD44 forms a family of cell surface proteins involved in cell migration, cell adhesion, tumor progression and metastatic spread (9-11). The CD44 gene is 50-60 kDa in size, located on chromosome 11p13, and is known to be composed of at least 20 exons. CD44v6 is an important isoform of CD44 family (12-14). Related studies have shown that the expression of CD44v6 correlates with tumor progression and metastasis development in several human malignancies, such as lung cancer, gastrointestinal cancer, breast cancer, and so on (10,13,15-17). However, reports on the relationship between CD44v6 expression and prognosis are contradictory. Some authors have reported that the CD44v6 is an independent prognostic factor (15,18), but others have failed to draw a similar conclusion (19,20). Therefore, we examined the expression of CD44v6 in T2-3N0M0 ESCC to elucidate its significance in clinical prognosis.
Materials and methods
Patients and tissues
The study was approved by the Research Ethics Committee of the Cancer Center of Sun Yat-Sen University. From January 1993 to August 2004, we enrolled 227 consecutive patients with stage IB-IIB node-negative ESCC who received surgical treatment with curative intent, and the resected specimens were assessed for CD44v6 expression level via immunohistochemistry (IHC). Patients were followed prospectively and their survival data was recorded through October 2009. Patients were included in this study based on the following eligibility criteria: (I) histopathologically proven ESCC; (II) disease stage of T2-3N0M0 based on the seventh edition of the American Joint Committee on Cancer (AJCC) staging system for esophageal cancer (21); (III) at least 15 lymph nodes to be removed for pathological evaluation; (IV) age at least 18 years; (V) no evidence of metastatic disease as determined by history, physical examination, and blood chemistry analysis or routine computed tomography; and (VI) no history of adjuvant therapy. Patients were excluded based on the following criteria: (I) history of previously treated cancer other than basal or squamous cell carcinoma of the skin; (II) history of preoperative chemotherapy and/or radiotherapy; (III) unknown cause of death in follow-up.
Immunohistochemistry (IHC)
Immunoperoxidase stain for CD44v6 (1:50 dilution; Novocastra Laboratories, Ltd., Newcastle upon Tyne, United Kingdom) was done on 4-μm-thick paraffin sections. The slides were deparaffinized in xylene then hydrated prior to antigen retrieval by microwaving in sodium citrate buffer (pH 6.0). The slides were then incubated with a peroxidase block and then the primary antibody. After a PBS wash, the slides were incubated first with the secondary antibody and then with 3,3'-diaminobenzidine, then counterstained with hematoxylin (Hematoxylin 7211; Richard-Allen Scientific, Kalamazoo, Michigan, United States of America). The peroxidase block, secondary antibody and 3,3'-diaminobenzidine were all obtained from the DakoCytomation EnVision System (Glostrup, Denmark). After a hematoxylin counterstain (Hematoxylin 7211; Richard-Allen ScientiWc, Kalamazoo, MI), the slides were coverslipped.
Immunohistochemical scoring
The immunohistochemical scoring of CD44v6 was performed using a semiquantitative system as demonstrated previously (7,15,22). Each slide was assigned a score: the score of tumor cell staining multiplied by the score of staining intensity. Tumor cell staining was assigned a score using a semiquantitative six-category grading system: 0, no tumor cell staining; 1, 1% to 10% of tumor cells staining; 2, 11% to 25% of tumor cells staining; 3, 26% to 50% of tumor cells staining; 4, 51% to 75% of tumor cells staining; 5, more than 75% of tumor cells staining. Stain intensity was assigned a score using a semiquantitative four-category grading system: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. Two experienced pathologists independently scored 400 ESCC samples including the cases used in this study blinded to clinical follow-up data. The complete score agreement between these two pathologists is 87% of cases, indicating that the scoring method was reasonably reproducible. A third blinded pathologist intervened and evaluated cases with different IHC scores. If the third pathologist agreed with one of the previous scorings, that score was used for analysis. For the cases in which three different scores were obtained, the three pathologists were asked to reach agreement on the case.
Selection of cut-off score
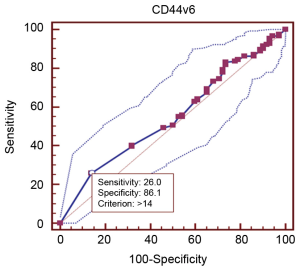
The cut-off scores for CD44v6 expression were selected based on receiver operating characteristic (ROC) curve analysis. The sensitivity and specificity for the outcome under study were plotted, thus generating a ROC curve. The score closest to the point with both maximum sensitivity and specificity [i.e., the point (0.0, 1.0) on the curve] was selected as the cut-off score leading to the greatest number of tumors classified correctly as having or not having the clinical outcome. The area under the ROC curve (AUC) was calculated to estimate the discriminatory power of CD44v6 over the entire range of scores for overall survival. Both generation and analysis of the ROC curve were performed by a MedCalc statistical software package 11.0.1 (MedCalc Software bvba, Belgium).
Statistical analysis
Associations between categorical variables were analyzed using the Chi-square test. Survival curves were calculated by the Kaplan-Meier method and were compared by the log-rank test. Time to event (death) was calculated from date of surgery to date of event. In event-free subjects, the time variable was censored at date of last follow-up. Multivariate analysis of prognostic factors was performed using Cox’s regression model. A significant difference was defined as a two-tailed P-value of less than 0.05. All of the statistical analyses were performed using the SPSS 13.0 for Windows software system (SPSS Inc, Chicago, IL, USA).
Results
Characteristics of all patients and expression of CD44v6
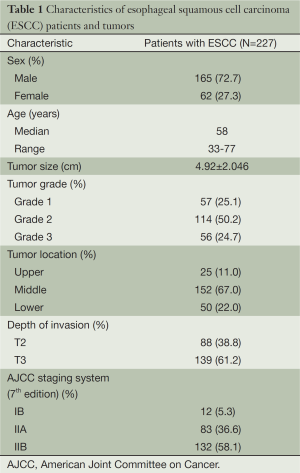
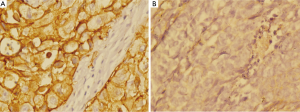
Table 1 provided all the patients’ clinicopathological features. Among the 227 patients included in this study, 162 (72.7%) were male patients and 62 (27.3%) were female patients. The median age was 58 years (range from 33 to 77 years). In cancer cells, it was generally the cytoplasm and membrane that revealed various intensity of positive reaction for CD44v6 (Figure 1). According to the ROC curve in our study (Figure 2), threshold value of 14 was the closest to the point with both maximum sensitivity and specificity, and thereby selected as the cut-off score. The AUC of our ROC curve analysis was 0.550 [95% confidence interval (CI): 0.483-0.616]. Patients were thus divided into two groups, namely high expression group (n=50) and low expression group (n=177).
Full table
Correlation between the expression of CD44v6 and clinicopathological features
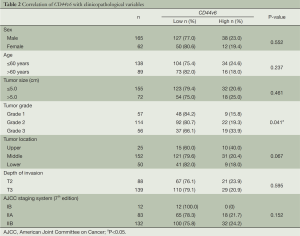
The association between CD44v6 expression and clinicopathological variables of ESCC patients is shown in Table 2. Low CD44v6 expression was found in 66.1% of Grade 3 of ESCC, significantly lower than that of Grade 1 and Grade 2 of ESCC. There are no significant correlations between CD44v6 expression and other clinicopathological parameters including gender, age, tumor size, tumor location, depth of invasion and pathological stage based on the seventh edition of AJCC staging system (21).
Full table
CD44v6 expression and survival
At the time of data analysis (October 2009), with a median follow-up of 32 months (range, 5-138 months), 72 patients (31.7%) remained alive and 155 patients (68.3%) died from cancer-related causes. The 1-, 3- and 5-year overall survival probabilities for the entire group were 58%, 39% and 33%, respectively.
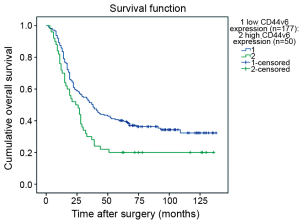
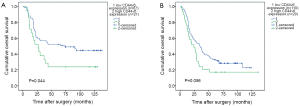
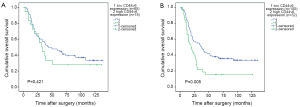
The Kaplan-Meier survival curves (Figure 3) showed that patients with low CD44v6 expression experienced significantly better post-operative survival than those with high CD44v6 expression (P=0.009). In further stratified analysis split by depth of invasion (Figure 4), CD44v6 expression had a statistically significant influence on the survival of patients with T2 diseases (P=0.044) rather than on the survival of those with T3 lesions (P=0.056). Furthermore, the stratified analysis split by pathological stage based on the new staging system (Figure 5) revealed that the prognostic significance of CD44v6 expression remained on the survival of patients with stage IIB ESCC (P=0.005) but not on the survival of those with IIA diseases (P=0.421). Patients with stage IB ESCC were not included in this analysis in view of the small sample size.
We performed analysis using the Cox proportional hazards model to identify factors involved in OS of T2-3N0M0 ESCC patients (Table 3). The univariate analysis showed that depth of invasion and CD44v6 expression were significant prognostic indicators for OS, and thereby selected as the parameters to be included in the same Cox regression model. Further multivariate analysis also confirmed that CD44v6 expression (relative risk, 1.639; 95% CI: 1.142-2.354, P=0.007) and depth of invasion (relative risk, 1.487; 95% CI: 1.063-2.080, P=0.020) were independent prognostic factors.
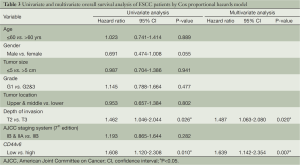
Full table
Discussion
Numerous studies have been performed in order to elucidate the role of CD44v6 in ESCC; however, data for this prognostic marker in ESCC are quite controversial (15,18-20). One problem faced by researchers is the determination of the extent of tumor IHC positivity for CD44v6 which is clinically and biologically relevant. Several previous studies had applied different scoring systems and predetermined cut-off scores which might be set arbitrarily. This may primarily be responsible for the contradictory results of these studies evaluating CD44v6 and its prognostic value in ESCC. Therefore, our study utilized a reproducible scoring method which took both staining percentage and intensity into account, and selected the cut-off score based on ROC curve analysis for CD44v6 expression evaluation, where the score closest to the point with both maximum sensitivity and specificity was chosen. This method allowed the greatest number of tumors to be correctly classified as carrying or not carrying the clinical outcome (23). In addition, all patients contained in our series were all free of lymph node metastasis and received no other therapy before or after the resection.
In the present study of T2-3N0M0 ESCC patients, high expression of CD44v6 was significantly correlated with the worse post-operative survival rate, both by univariate and multivariate analysis, which is consistent with the reported findings. Nozoe et al. (24) reported that the over-expression level of CD44v6 in ESCC patients is significantly correlated with the higher proportions of the incidence of lymph node metastasis, lymphatic permeation, blood vessel invation, more advanced stage and therefore poorer prognosis. Similarly, Shen et al. (25) received a comparable conclusion that CD44v6 expression in ESCC was significantly higher than that in adjacent normal tissue and was correlated with distant metastasis and TNM stage.
It has been known that well differentiation of ESCC which is deeply related to apoptosis to the tumor cells is likely to influence the less invasive potential of ESCC (26,27). Several investigations suggest the correlation of CD44v6 with the histologic grade in SCC of the head and neck (28,29). In our study, we draw a similar conclusion that the proportion of low CD44v6 expression was found significantly lower in Grade 3 of ESCC, than that of Grade 1 and Grade 2 of ESCC, which was consistent with Shen et al. (25).
Moreover, CD44v6 expression has been reported to be an indicator to predict the sensitivity to neoadjuvant chemotherapy in cervical carcinoma (30); hence, an assessment of an IHC CD44v6 expression might possibly be an auxiliary information regarding the response of ESCC to the adjuvant therapies. Also, Koyama et al. (31) reported that the expression of CD44v6 was significantly reduced in the irradiated primary ESCC, which might imply that CD44v6 could be a factor on the response of ESCC to radiotherapies.
In conclusion, the study elucidated that CD44v6 might be correlated with the histologic grade and its high expression may be a potential worse prognosis indictor for the patients with T2-3N0M0 ESCC, especially for the patients with T2 lesions or stage IIB diseases. However, there are still many unanswered questions regarding CD44v6 expression in ESCC. Additional studies with randomization and longer follow-up are needed for the establishment of more effective management plan, which will aid in improving prognosis.
Acknowledgements
This study was supported by the grant from the Fundamental Research Funds for the Central Universities Youth Training Plan of Sun Yat-Sen University, (No. 10ykpy38), the Research Award Fund for Outstanding Young researchers in Sun Yat-sen Cancer Center (No. 303045172006; No. 303045172005), and Science and Technology Planning Project of Guangdong Province (No. 2011B031800220). The authors confirmed independence of the sponsors; the content of the article has not been influenced by the sponsors.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-41. [PubMed]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001;37 Suppl 8:S4-66. [PubMed]
- Ekman S, Dreilich M, Lennartsson J, et al. Esophageal cancer: current and emerging therapy modalities. Expert Rev Anticancer Ther 2008;8:1433-48. [PubMed]
- Lambert R. Endoscopy in screening for digestive cancer. World J Gastrointest Endosc 2012;4:518-25. [PubMed]
- Situ DR, Hu Y, Zhu ZH, et al. Prognostic relevance of β-catenin expression in T2-3N0M0 esophageal squamous cell carcinoma. World J Gastroenterol 2010;16:5195-202. [PubMed]
- Yu Y, Wang B, Zhang K, et al. High expression of lysine-specific demethylase 1 correlates with poor prognosis of patients with esophageal squamous cell carcinoma. Biochem Biophys Res Commun 2013;437:192-8. [PubMed]
- Martin TA, Harrison G, Mansel RE, et al. The role of the CD44/ezrin complex in Cancer metastasis. Crit Rev Oncol Hematol 2003;46:165-86. [PubMed]
- Heider KH, Kuthan H, Stehle G, et al. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother 2004;53:567-79. [PubMed]
- Barbour AP, Reeder JA, Walsh MD, et al. Expression of the CD44v2-10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res 2003;63:887-92. [PubMed]
- Ponta H, Wainwright D, Herrlich P. The CD44 protein family. Int J Biochem Cell Biol 1998;30:299-305. [PubMed]
- Afify A, Purnell P, Nguyen L. Role of CD44s and CD44v6 on human breast Cancer cell adhesion, migration, and invasion. Exp Mol Pathol 2009;86:95-100. [PubMed]
- Jijiwa M, Demir H, Gupta S, et al. CD44v6 regulates growth of brain tumor stem cells partially through the AKT-mediated pathway. PLoS One 2011;6:e24217. [PubMed]
- Situ D, Long H, Lin P, et al. Expression and prognostic relevance of CD44v6 in stage I non-small cell lung carcinoma. J Cancer Res Clin Oncol 2010;136:1213-9. [PubMed]
- Xin Y, Grace A, Gallagher MM, et al. CD44V6 in gastric carcinoma: a marker of tumor progression. Appl Immunohistochem Mol Morphol 2001;9:138-42. [PubMed]
- Yu P, Zhou L, Ke WF, et al. Clinical significance of pAKT and CD44v6 overexpression with breast cancer. J Cancer Res Clin Oncol 2010;136:1283-92. [PubMed]
- Zhou DX, Liu YX, Xue YH. Expression of CD44v6 and Its Association with Prognosis in Epithelial Ovarian Carcinomas. Patholog Res Int 2012;2012:908206.
- Ma W, Deng Y, Zhou L. The prognostic value of adhesion molecule CD44v6 in women with primary breast carcinoma: a clinicopathologic study. Clin Oncol (R Coll Radiol) 2005;17:258-63. [PubMed]
- Morris SF, O’Hanlon DM, McLaughlin R, et al. The prognostic significance of CD44s and CD44v6 expression in stage two breast carcinoma: an immunohistochemical study. Eur J Surg Oncol 2001;27:527-31. [PubMed]
- Hsu PK, Wu YC, Chou TY, et al. Comparison of the 6th and 7th editions of the American joint committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg 2010;89:1024-31.
- Zhu ZH, Sun BY, Ma Y, et al. Three immunomarker support vector machines-based prognostic classifiers for stage IB non-small-cell lung cancer. J Clin Oncol 2009;27:1091-9. [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [PubMed]
- Nozoe T, Kohnoe S, Ezaki T, et al. Significance of immunohistochemical over-expression of CD44v6 as an indicator of malignant potential in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2004;130:334-8. [PubMed]
- Shen WD, Ji Y, Liu PF, et al. Correlation of E-cadherin and CD44v6 expression with clinical pathology in esophageal carcinoma. Mol Med Rep 2012;5:817-21. [PubMed]
- Ohbu M, Saegusa M, Okayasu I. Apoptosis and cellular proliferation in oesophageal squamous cell carcinomas: differences between keratinizing and nonkeratinizing types. Virchows Arch 1995;427:271-6. [PubMed]
- Nozoe T, Korenaga D, Itoh S, et al. Clinicopathological significance of pRb2/p130 expression in squamous cell carcinoma of the esophagus. J Cancer Res Clin Oncol 2002;128:691-6. [PubMed]
- Fonseca I, Pereira T, Rosa-Santos J, et al. Expression of CD44 isoforms in squamous cell carcinoma of the border of the tongue: A correlation with histological grade, pattern of stromal invasion, and cell differentiation. J Surg Oncol 2001;76:115-20. [PubMed]
- Rodrigo JP, Domínguez F, Alvarez C, et al. Clinicopathologic significance of expression of CD44s and CD44v6 isoforms in squamous cell carcinoma of the supraglottic larynx. Am J Clin Pathol 2002;118:67-72. [PubMed]
- Costa S, Terzano P, Bovicelli A, et al. CD44 isoform 6 (CD44v6) is a prognostic indicator of the response to neoadjuvant chemotherapy in cervical carcinoma. Gynecol Oncol 2001;80:67-73. [PubMed]
- Koyama S, Maruyama T, Adachi S. Expression of epidermal growth factor receptor and CD44 splicing variants sharing exons 6 and 9 on gastric and esophageal carcinomas: a two-color flow-cytometric analysis. J Cancer Res Clin Oncol 1999;125:47-54. [PubMed]


