Reliability of intramyocardial electrogram for the noninvasive diagnosis of acute allograft rejection after heart transplantation in rats
Introduction
Heart transplantation is the treatment of choice for eligible patients with end-stage heart diseases, and acute rejection after transplantation can severely threaten patient survival (1,2). The development of new transplantation techniques and immunosuppressants has greatly improved heart transplantation efficacy. However, studies on the diagnosis of allograft rejection (AR) based on clinical manifestations, surface electrocardiograms, X-ray, cellular and humoral immune responses, and cardiac ultrasounds do not yield satisfactory results. According to an international multicenter study (Cardiac Transplant Research Database) (1), 15% of all deaths associated with heart transplantation result from allograft. AR occurs most frequently during the 1st or 2nd postoperative month (2) and can cause cardiac function deterioration and chronic rejection, significantly affecting the long-term survival of heart transplant recipients. Accordingly, a delay in AR detection can have severe consequences.
Although endomyocardial biopsy (EMB) displays good diagnostic accuracy and uniform diagnostic criteria (3), making it the current gold standard for AR diagnosis after heart transplantation, its use retains several inherent disadvantages. AR can be focal, and local biopsy may fail to represent the overall cardiac condition. Humoral-mediated AR may be mild in manifestation but lead to severe consequences (2). EMB is expensive, difficult to perform on pediatric patients, can damage the cardiac conduction system, and can induce arrhythmia, tricuspid regurgitation (3), cardiac perforation, or even death (2). Dynamic surveillance is not possible with EMB, and diagnosis is not immediate. The interpretation of EMB results is subject to inter- and intra-observer variation, and thus the consistency and objectivity of the results are impaired. There is, therefore, a pressing need for a non-invasive, fast, sensitive, specific, and dynamic method to replace or supplement EMB.
Surface electrocardiogram has also been employed in monitoring immunological rejection. However, its method specificity is extremely low, due to influences from many external factors, and surface electrocardiograms are poorly correlated to histological results (4). As a relatively stable tool capable of accurately reflecting electrophysiological changes of focal cardiac muscles, intramyocardial electrogram (IMEG) has emerged as a promising method for AR diagnosis after heart transplantation. Most studies on the utility of IMEG have focused on the diagnostic value of the QRS amplitude of autonomous IMEG and the maximum slope of the descending T wave of the ventricular evoked response (VER or paced IMEG) (5-7). Such studies indicate that IMEG offers non-invasive, efficient, safe, and convenient AR surveillance (7,8), and give high credence to using the QRS amplitude and the maximum slope of the descending T wave as noninvasive markers. However, controversy exists as to their actual usefulness in AR diagnosis (9). In addition, no systematic or statistical evaluation has been performed with regard to the utility of IMEG for AR surveillance.
To address this issue, here we monitored changes in the QRS amplitude of the autonomous IMEG and the maximum slope of the descending T wave of VER in an abdominal heart transplantation model in rats. We analyzed and compared the reliability of both indices in AR diagnosis after heart transplantation, using interval estimation and receiver operating characteristic (ROC) curve analysis.
Methods
Animals
Eight-week-old male Lewis rats (n=10) and Sprague Dawley rats (n=30) with an average body weight of 280 g (250-300 g) were obtained from the experimental animal center of Nantong University, and were used as recipients and donors. Epicardial electrodes were implanted at the right ventricular outflow tract, left ventricular free wall, and left ventricular apex. All surgical interventions and postoperative animal care were performed in accordance with the National Institutes of Health Guide-lines for the Care and Use of Laboratory Animals (National Research Council, 1996, USA) and were approved by the Chinese National Committee to the Use of Experimental Animals for Medical Purposes, Jiangsu Branch. All procedures were performed on animals in an unconscious state. All efforts were made to minimize the number of animals used and their suffering.
Acquisition of autonomous and paced intramyocardial electrograms
Autonomous IMEG and VER were recorded at days 3, 5 and 7 after heart transplantation, at the same time each day. At least 50 autonomous and paced QRS complexes were recorded at each time point, and at least ten consecutive QRS complexes per minute of a 5-min period of continuous recording were selected for data analysis.
Observation indices
The QRS amplitude of IMEG was defined as the voltage (mV) of the QRS complex from trough to peak, and was calculated with a biomedical signal acquisition and processing system (PCLAB-UE, Beijing Microsignalstar, China). The maximum slope of the descending T wave of VER was obtained directly from PCLAB-UE (Figure 1). All data collected during each recording were averaged.
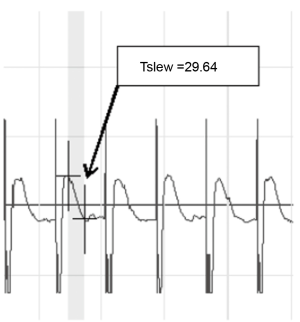
Experimental endpoints
Day 7 after operation was chosen as the experimental endpoint for the ten isograft group syngeneic recipients. The 30 allograft group allogeneic recipients were randomized into three groups (n=10) with experimental endpoints of days 3, 5 and 7 after operation, respectively. When animal subjects were eliminated before their experimental endpoints for various reasons, new matched rats were introduced to maintain a constant number of animals within each group.
Pathological examination
At the end of the experimental endpoints, laparotomy was performed under general anesthesia and the transplanted heart was removed while still beating. The heart was fixed in 10% formaldehyde for 24 h. Subsequently, two myocardial sections of ~1 mm thick were cut, embedded in paraffin, and stained by hematoxylin and eosin stain. Diagnosis of rejection was established by the same group of pathologists in a blind fashion, according to the International Society of Heart and Lung Transplantation (ISHLT) system for rejection grading (10). Rats with a rejection grade of II or above were rejection positive, while those with grades of I or 0 were rejection negative.
Statistical analysis
Results are expressed as the means ± standard deviations. Data analysis was performed using Stata 10.0 with the significance level set at α=0.05. P-values
Results
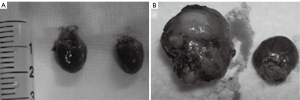
Values day 2 after operation were designated as baseline and set to 1. The relative value at each time point was calculated as the absolute value at each time point divided by the absolute value at day 2. The syngeneic hearts were visually similar in size to the autogenous hearts, while the allogeneic hearts were significantly increased in size (Figure 2). Pathological examination at the experimental endpoints revealed no cases of rejection in the isograft group, but 19 positive and 11 negative cases in the allograft group. In the allograft group, rejection began at day 3 with a rejection grade of 2 or 3, and all rejection episodes were of grade 3-4 at day 7 (Figure 3). Evaluation of the diagnostic cutoff values and diagnostic values was performed on the allograft group, and the pathological results were chosen as the gold standard.
QRS amplitude of the autonomous intramyocardial electrogram and the maximum slope of the descending T wave of ventricular evoked response
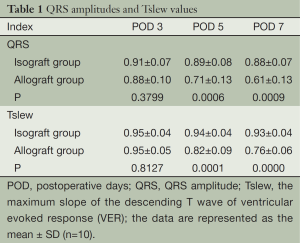
The autonomous QRS amplitudes and the maximum slope of the descending T wave values of VER are shown in Table 1, respectively. No significant change in the QRS amplitude or the maximum slope of the descending T wave occurred in the isograft group during the study period. In contrast, these two indices were significantly decreased at the experimental endpoint in the allograft group compared with baseline values.
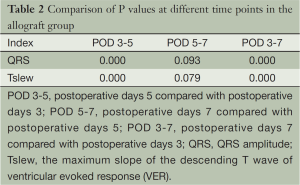
Comparisons of the QRS amplitudes between groups at various time points after surgery are listed in Table 1. A group t-test was performed on data collected at days 3 and 5, and a rank-sum test was performed on values obtained at day 7 due to heterogeneity of variance. We observed no marked difference between the QRS amplitudes on the postoperative day 3 of the two groups, but significant differences were noted in the subsequent two time points. Comparisons of the maximum slope of the descending T wave values between groups after surgery showed a similar pattern (Table 1). Comparisons of the allograft group QRS amplitudes and the maximum slope of the descending T wave values at different time points are summarized in Table 2. Except for those between days 5 and 7, comparisons between all time points showed significant differences in the QRS amplitudes and the maximum slope of the descending T wave values.
Full table
Full table
Correlation of the QRS amplitude and the maximum slope of the descending T wave
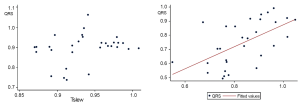
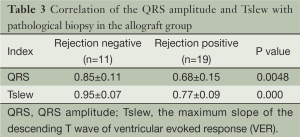
A linear correlation was observed between the two indices of the allograft group (r=0.5816, P=0.0007), but not of the isograft group (r=0.2639, P=0.1587) (Figure 4). The correlation of the QRS amplitude and the maximum slope of the descending T wave value with pathological results in the allograft group are shown in Table 3. Of note, allogeneic recipients that were rejection positive had remarkably lower QRS amplitudes and the maximum slope of the descending T wave values than those negative in the pathological biopsy.
Full table
Evaluation of diagnostic values of the QRS amplitude and the maximum slope of the descending T wave
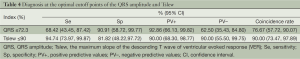
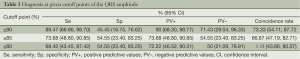
A cutoff point separates positive from negative values. The specificity (Sp), sensitivity (Se), positive (PV+) and negative (PV–) predictive values and the coincidence rate of the QRS amplitude and the maximum slope of the descending T wave at their corresponding optimal cutoff points are shown in Table 4. Diagnoses at various cutoff points of the QRS amplitude and the maximum slope of the descending T wave are given in Tables 5 and 6, respectively.
Full table
Full table

Full table
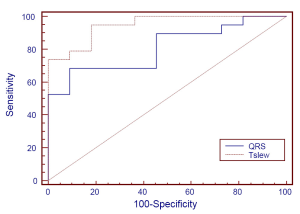
At a cutoff point of 92% (≤92% considered positive), the maximum slope of the descending T wave had 100% sensitivity, 63.64% specificity, and 82.61% positive and 100% negative predictive values. At the optimal cutoff point of 90%, the maximum slope of the descending T wave had 94.74% sensitivity, 81.82% specificity, and 90% positive and 90% negative predictive values. At the optimal cutoff point of 72.3%, QRS had 68.42% sensitivity, 90.91% specificity, and 92.86% positive and 62.50% negative predictive values. The area under the ROC curve of the QRS amplitude (0.8086; 95% CI: 0.65319, 0.96404) significantly differed (χ2=4.32, P=0.0377) from that of the maximum slope of the descending T wave (0.9474; 95% CI: 0.87528, 1.000) (Figure 5).
Discussion
Previous studies on IMEG have been limited to the empirical selection of one or several diagnostic criteria (or threshold values/cutoff points) to calculate the sensitivity, specificity, and other indices. Diagnostic value evaluation was reportedly based on point rather than interval estimation, overlooking the effects of sampling error or sample rate. Since these indices are related to the selected diagnostic criteria or threshold values, different diagnostic criteria may yield different results for the diagnostic value of IMEG. Therefore, it is insufficient to assess the diagnostic value of IMEG simply based on the empirical selection of diagnostic criteria.
In the present study, we used ROC analysis to assess the diagnostic value of the QRS amplitude and the maximum slope of the descending T wave. At the optimal cutoff point of the maximum slope of the descending T wave, we obtained a sensitivity and specificity of 94.74% and 81.82%, respectively, and a 90% positive and negative predictive value and coincidence rate. At the optimal cutoff point of the QRS amplitude, the sensitivity was 90.91% and the specificity was only 68.42%, while the positive and negative predictive values and coincidence rate were 92.86%, 62.50%, and 76.67%, respectively. The area under the ROC curve of the QRS amplitude of autonomous IMEG was 0.8086, suggesting that the QRS amplitude is a moderately reliable diagnostic criterion. In contrast, the area under the ROC curve of the maximum slope of the descending T wave was 0.9474, suggesting that the maximum slope of the descending T wave is a highly reliable diagnostic criterion. The significant difference between these indices is attributable to the higher diagnostic coincidence rates of the maximum slope of the descending T wave at its various selected cutoff points. While both have the same sensitivity level, the maximum slope of the descending T wave usually has a higher specificity and lower false positive rate than the QRS amplitude.
The QRS amplitude and the maximum slope of the descending T wave of VER in both groups declined progressively after transplantation. In allograft recipients, these values were correlated to the pathological findings, and were markedly lower in patients positive in pathological examination, confirming that changes in the QRS amplitude and the maximum slope of the descending T wave were associated with AR. These results seem to suggest that the QRS amplitude and the maximum slope of the descending T wave followed similar patterns of change. However, correlation analysis revealed that the indices were linearly correlated in the allograft group, but not in the control. Interference from factors other than AR may produce different effects on the two indices. However, rejection seemed to synchronize the variation of both indices, which might contribute to the better performance of the maximum slope of the descending T wave versus the QRS amplitude in AR surveillance.
One possible reason for the false positive diagnosis by the QRS amplitude is poor electrode contact. We eliminated three rats (one syngeneic and two allograft recipients) from the study for this reason. The QRS amplitudes in these three rats were significantly reduced to 30-60% of the baseline values, suggesting that AR occurred according to the general criteria. However, the autopsy results showed the sliding of at least one cardiac electrode to the muscular layer of the abdominal wall, indicating that the marked decline in the autonomous IMEG in the three rats may have been derived from the cardiac electrical conduction of the transplanted heart to the body surface. For example, the QRS amplitude was reduced by >30% at day 3, while the maximum slope of the descending T wave was reduced by in situ. This suggests both the possibility of false positive diagnosis by the QRS amplitude, and also that this misjudgment could be eliminated when the maximum slope of the descending T wave was used alone or in combination with the QRS amplitude.
When using ≤92% as the positive cutoff point, the maximum slope of the descending T wave yielded a high sensitivity of 100% and a specificity of 63.64%, suggesting that AR should be suspected and the maximum slope of the descending T wave could be used as a sensitive index for screening AR. When using ≤85% as the positive cutoff point, the maximum slope of the descending T wave gave a higher specificity of 90.91% and a slightly lower sensitivity of 78.95%, indicating a high probability of AR. When the optimal positive cutoff point (≤90%) was used, the maximum slope of the descending T wave provided a satisfactory sensitivity, specificity, and coincidence rate. Therefore, if EMB biopsy is not indicated clinically, AR could also be diagnosed when the optimal positive cutoff point of the maximum slope of the descending T wave is met.
Taken together, these results indicate that the QRS amplitude of IMEG and the maximum slope of the descending T wave of VER can be used as noninvasive tools for AR diagnosis after heart transplantation, and that the maximum slope of the descending T wave is more reliable in AR surveillance than the QRS amplitude. The use of such indices may minimize the need for EMB or serve as a useful supplement to EMB. There are several limitations to our study. No correlation has been done between IMEG, gross pathology and microscopic pathology. We did not examine the physiological and ultrastructural mechanisms responsible for IMEG changes in response to AR, or the changes in the QRS amplitude of IMEG and the maximum slope of the descending T wave after AR disappearance following immunosuppressive treatment. Modified splint tube technique can be used to get heart transplantation models easily (11). Further studies addressing these issues are warranted.
Acknowledgements
This work was supported by the “Top Six Types of Talents” Financial Assistance of Jiangsu Province Grant (WS-059) and the Science Foundation of Nantong City Grant (K2010055).
Disclosure: The authors declare no conflict of interest.
References
- Kubo SH, Naftel DC, Mills RM Jr, et al. Risk factors for late recurrent rejection after heart transplantation: a multiinstitutional, multivariable analysis. Cardiac Transplant Research Database Group. J Heart Lung Transplant 1995;14:409-18. [PubMed]
- Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation: pathologic observations and clinical implications. J Heart Transplant 1989;8:430-43. [PubMed]
- Zerbe TR, Arena V. Diagnostic reliability of endomyocardial biopsy for assessment of cardiac allograft rejection. Hum Pathol 1988;19:1307-14. [PubMed]
- Keren A, Gillis AM, Freedman RA, et al. Heart transplant rejection monitored by signal-averaged electrocardiography in patients receiving cyclosporine. Circulation 1984;70:I124-9. [PubMed]
- Warnecke H, Müller J, Cohnert T, et al. Clinical heart transplantation without routine endomyocardial biopsy. J Heart Lung Transplant 1992;11:1093-102. [PubMed]
- Müller J, Eubel A, Dandel M, et al. Non-invasive monitoring of rejection after cardiac transplantation. The method and retrospective analysis of data on 734 patients. Dtsch Med Wochenschr 2001;126:1223-8. [PubMed]
- Jia YX, Meng X, Sun LB, et al. Using intramyocardial electrograms combined with other noninvasive methods for monitoring acute rejection following human heart transplantation. Chin Med J (Engl) 2009;122:136-9. [PubMed]
- Knosalla C, Grauhan O, Muller J, et al. Intramyocardial electrogram recordings (IMEG) for diagnosis of cellular and humoral mediated cardiac allograft rejection. Ann Thorac Cardiovasc Surg 2000;6:89-94. [PubMed]
- Bainbridge AD, Cave M, Newell S, et al. The utility of pacemaker evoked T wave amplitude for the noninvasive diagnosis of cardiac allograft rejection. Pacing Clin Electrophysiol 1999;22:942-6. [PubMed]
- Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 1990;9:587-93. [PubMed]
- Li C, Luo L, Lu J, et al. A modified splint tubing technique for heterotopic heart transplantation in mouse. Transpl Immunol 2011;25:82-7. [PubMed]


