Single-lung and double-lung transplantation: technique and tips
Introduction
After the first successful single-lung transplantation in 1983, the Toronto team described the first successful double-lung transplantation in 1988 (1,2). The lungs were implanted “en-bloc” through a median sternotomy, three anastomoses were performed: one tracheal, one on the pulmonary artery (PA) and one on the left atrium. This procedure was proved to be technically demanding and likely to lead to impaired airway anastomosis healing (3).
It has been therefore abandoned in favor of the bilateral sequential technique where one single lung transplantation is performed after the other, with or without associated cardiopulmonary bypass (CPB), as described by the Foch and St Louis teams (4-6).
Anesthesiologic preparation (Figure 1)
The anesthetic preparation of the recipient is the first step of the surgery, and needs a well-trained team, as well as specific devices.
In addition to the usual induction tools and double-lumen endotracheal tube (Carlens), the anesthesiologist should prepare an autologous blood recovery system such as Cell Saver®, a Swan-Ganz catheter for monitoring the pulmonary arterial pressure, cardiac index and SVO2. A deep venous catheter is placed in a jugular vein (4 lines), as well as a radial arterial line for monitoring of the blood pressure, and two peripheral venous catheters. Depending on the experience of the anesthesiologist a probe for trans-esophageal echocardiography is placed, or a nasogastric tube if not. If cardio-pulmonary bypass is expected to be required during surgery, the Swan-Ganz catheter is not mandatory and visual monitoring of heart function can be performed by trans-esophageal echography exclusively. A bladder catheter is also placed, allowing monitoring of diuresis and body temperature.
A defibrillator should be present in the operating theatre, as well as the material necessary to put a chest tube in case of pneumothorax during the induction (especially for COPD patients). Adequate amount of blood is ordered depending on the expected difficulty with the surgery [patient history and potential extracorporeal membrane oxygenation (ECMO)] and the medications taken by the recipient.
The patient is put to sleep only after the procurement team has accepted the lungs. The thoracic surgeon should have arrived by this point. If a patient has pre-operative pulmonary hypertension, a peripheral venoarterial ECMO may be necessary before induction of anesthesia. If not, the team must stay vigilant and be prepared to place the ECMO at any time for hemodynamic assistance in emergency conditions.
The whole preparation takes from one to two hours and must be planned during the initial organization of the lung transplantation.
Installation and surgical approach
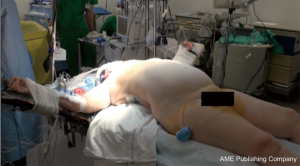
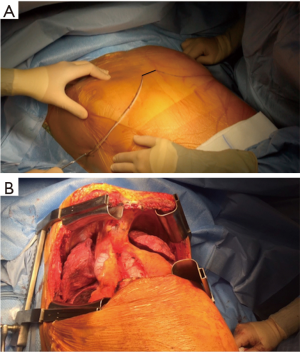
The patient is placed either with his arms spread, or crossed above his head (Figure 2). Initially a bilateral anterior thoracotomy in the fourth or fifth inter-costal space was performed, with an additional transversal sternotomy, realizing the clamshell incision. Because of the complications with this approach, such as pseudo-arthrosis, sternal infection, alteration of respiratory mechanics or dysfunction of the phrenic nerves, bilateral thoracotomy without sternal section is now performed whenever possible (Figures 3 and 4A). While the anterior thoracotomy is performed, the sub-cutaneous and breast tissue can be fixed upward to the operative field in case it is too voluminous (Figure 4B).
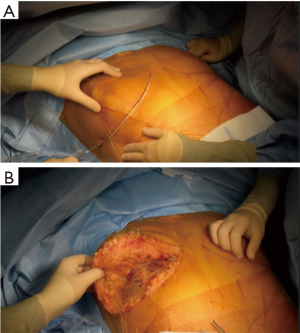
A clamshell approach is performed at the beginning of the lung transplant when a shrunken surgical field exposure is expected, as in patients with severe pulmonary fibrosis. Otherwise, it can be performed during the procedure if the patient becomes hemodynamically unstable, if unexpected pleural adhesions are found or hilum dissection is difficult. The need for ECMO does not necessarily require to split the sternum, as the surgeon has access to the descending aorta through the left thoracotomy, and the right atrium on the other side for cannulation.
Other approaches have been advocated:
- Lateral or posterolateral thoracotomy when difficult dissection is expected. But the patient then needs to be positioned a second time for the other side. This approach was used in the 2000’s for single lung transplantation;
- Median sternotomy when no pleural adhesions are expected and probability of central CPB is high, in primary pulmonary hypertension for example;
- Video-assisted anterior thoracotomy has been described by the Hanover team (7).
Surgical technique
Anesthesia begins after the procurement team has confirmed the acceptability of the donor lungs. From this moment, everything must be done to shorten the cold ischemic time as much as possible. The native lung and the hilum are dissected before the procurement team arrives. The PA clamping test is done as well at that point. Explantation of the lung on the other hand, is typically done only when the donor lungs are on site.
The first side implanted depends on the pre-operative examinations. If there is no dominant lung in the recipient, the first lung implanted is usually the right one. Because of anatomy the implantation is usually easier and quicker on this side. Bilateral lung transplantation is the association of two single lung transplantations.
We will describe the right lung transplantation in detail.
Lung dissection
The first step is to free the native lung from the parietal pleura. In case of history of lung surgery, pneumothorax and chest tube, pleural symphysis or suppurative and chronic infectious lung disease, it can be challenging.
Single-lung ventilation should be avoided as long as possible, or associated with clamping of the PA, to prevent shunt and hypoxemia. Traction on the hilum should also be avoided, as well as lung injury, to prevent bleeding and hemodynamic instability. Special care should be given in order to avoid injury to any recurrent or phrenic nerve.
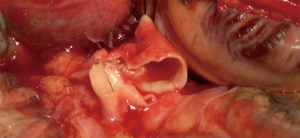
The aim of hilar dissection is to individualize each structure (bronchus, PA and left atrium) in order to be able to clamp these structures to perform safely the anastomoses distally from the clamp (Figure 5).

The pulmonary ligament is divided until the inferior pulmonary vein is reached. The veins are then freed from the surrounding pleura, which is pushed towards the lung, for better visualization and section of the adherences. The PA is then divided up until its first branch. A loop encircling the superior pulmonary vein first and then pulled downward can be helpful for exposure.
The veins are also encircled with loops and gently pulled, allowing the surgeon to have a good view on the pericardium, which is then opened with drainage of clear pericardial fluid. The easiest opening spot is located on the anterior and inferior side of the inferior vein. A dissector can be used to help completing the so-called pericardial window while dividing the pericardium around the whole hilum. Careful coagulation of vessels coming from the sub-carinal space should be done while freeing the upper part of the upper pulmonary vein. The section of the posterior part of the pericardium is performed in regard of the Haller pericardial sinus, while the veins are pulled upward.
The phrenic nerve should be carefully preserved during the whole procedure; it is left a few millimeters above the pericardial opening. The anterior edges of the pericardial window are suspended using stiches to facilitate the exposure of the hilum, and avoid incidental phrenic nerve injury.
Once the veins and the left atrium have been freed from all pericardial adherences, an extra length of atrial wall can be gained by dissecting the interatrial groove, according to the Sondergaard maneuver, to facilitate lateral clamping of the atrium. The dissection begins in the sulcus, just behind the connection to the superior vena cava with the right atrium, and is continued caudally where the sulcus becomes hardly noticeable.
The PA is freed from the pericardium by section of the fascias between them, going further than the vena cava, which is retracted carefully to avoid trauma of the phrenic nerve. The dissection is performed until the beginning of the mediastinal artery can be seen, as the PA clamping must be performed centrally. Sometimes when the first segmental artery of the right upper lobe originates early from the right PA, the later may be approached and controlled in the Theile pericardial sinus, in-between the superior vena cava and the ascending aorta.
Once all the vascular elements of the hilum are under control, a clamping test is performed on the PA. It allows the surgeon to check if the contralateral lung and the heart will provide sufficient and stable oxygenation and hemodynamics throughout the explantation and implantation of the other lung.
Saturation, blood pressure, pulmonary blood pressure and impact on the heart function (trans-esophageal echography) are monitored during the test. Though no specific time can be given, we usually perform a clamping test for ten minutes before explantation.
Active and repeated bronchial aspirations, pressure-controlled ventilation (volume 5 mL/kg, PEEP 5 cmH2O), administration of nitric oxide (increase of pulmonary arterial pressure), can improve unique lung ventilation while the contralateral lung is clamped.
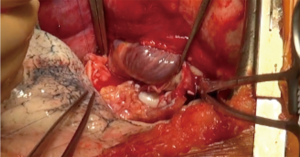
While the lead surgeon explant the lung and prepare the hilum for the implantation, the procurement team prepares the lung graft (Figure 6).

The lungs are preserved inflated either at 4 °C in three sterile bags containing respectively the preservation fluid, sterile physiological serum, and air or with a portable device such as the Organ care system (OCS, Transmedics™) for normothermic preservation (Figure 7). The lungs can be split either after the procurement in the donor hospital or immediately before the transplantation in the recipient hospital, depending on the team’s policy or the use of the OCS. The division of the two lungs is performed first on the left atrium in the middle of the posterior wall, than on the artery at the bifurcation, and finally on the trachea with a stapling device, leaving the right main bronchus and the trachea on one side, and the left main bronchus on the other. Retrograde flush via the pulmonary veins, useful to eliminate the last clots present in the graft, is performed as well at the site of procurement or prior to transplantation. The preservation solution is sampled routinely for microbiological analysis.

The preparation of the graft hilum has several goals:
- Resection of structures in excess: pericardium, fat, azygos vein, aortic arch, vena cava;
- Cutting the bronchus as short as possible. The donor bronchus is shortened with only one cartilage ring remaining proximal to the bifurcation of the upper lobe bronchus, or even at the level of the secondary carina, to prevent impaired bronchial anastomotic healing;
- Leave sufficient peribronchial fatty tissue to wrap the bronchial anastomosis once completed;
- Prepare an artery not too long, in order to avoid later obstructive plication;
- Conservation of the resected pieces of veins and artery in preservation fluid, to be used as patches to manage potential vascular anastomotic problems later during the procedure.
Explantation of the native lung should be performed after the arrival of the procurement team.
At this point, hilar dissection has already been done and the following steps therefore go fast. Usually the veins are sectioned first with a stapling device, followed by the artery. On the right side, the mediastinal artery is often stapled separately from the main PA. Vessels stumps should be left as long as possible to facilitate the anastomoses (Figure 8).

The bronchus is then divided above the origin for the upper lobe bronchus. Careful hemostasis is performed. From this point, the surgical field is “contaminated” and the Cell Saver device to recover red blood cells can no longer be used until the bronchus is closed.
The PA is gently pulled to allow the surgeon to put a clamp on the artery as far proximal behind the superior caval vein as possible. If a Swan-Ganz probe has been placed, the surgeon should check its position and if the catheter is in the clamping area, withdraw it.
The same maneuver is performed on the left atrium, to test the feasibility and safety of later clamping.
The recipient bronchus is prepared centrally while avoiding denuding it beyond the level of the planned anastomosis. This contributes to prevent ischemia of the bronchial anastomosis, even if the main risk factor for this complication lies at the level of the donor bronchus. Practically in some recipients such as those with cystic fibrosis, it is often necessary to remove bulky lymph nodes to facilitate the exposure and dissection of the vessels. Nevertheless, excessive lymphadenectomy should be avoided to prevent hardly controllable bleeding in the posterior mediastinum or some catastrophic complications such as esophageal perforation.
After the vascular clamps have been chosen, they are removed for the bronchial anastomosis, the first one performed. Prolene traction sutures are placed on the pericardium, and between the PA and the pericardium for a better exposure of the bronchus (Figure 9).

Pulmonary implantation
Cold gauze sponges are placed in the posterior aspect of the pleural cavity, before the graft is positioned there. We perform the bronchial anastomosis first, then the arterial one, and finally the atrial one. Some teams choose a different sequence, starting with the venous anastomosis, as it is the deepest, followed by the PA and finally the bronchus.
Bronchial anastomosis (Figure 10)
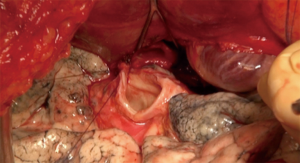
Great care must be taken while performing this anastomosis, as complications, especially ischemic ones, are associated with higher morbidity and mortality rates (12). A flap, either from the recipient surrounding fatty tissues, an inter-costal muscle, or using the donor pericardium, reinforces the anastomosis. The right bronchus is anatomically shorter and wider than the left one, but there are otherwise no differences between the two anastomoses.
Different techniques can be used to perform the bronchial anastomosis (13-16):
- One continuous running suture on the whole circumference of the anastomosis;
- A posterior running suture on the membranous part of the bronchus, and separate stitches on the cartilaginous part;
- A telescopic suture when the diameters of the two bronchi are too different and major discrepancy is expected. This technique carries a higher rate of anastomotic complications.
We usually perform an end-to-end technique with a double needle slowly absorbable monofilament (PDS 3.0), starting the running suture at the upper part of the membranous wall of the bronchi consisting of the posterior aspect of the anastomosis. We use the same suture for the anterior cartilaginous aspect of the anastomosis and tie the two ends of the stitch in the middle. The chosen flap is then placed around the suture to prevent arterio-bronchial fistula (Figure 11).

Re-anastomosis of the bronchial arteries has been described to increase vascularization of the sutured bronchus, but it is a long and complex procedure, without strong evidence of its benefit to prevent later complications (18,19). It is mostly not used anymore.
Arterial anastomosis (Figure 12)
The arterial anastomosis is the second one we perform. A vascular clamp must be placed as far as possible on the proximal part of the PA, while the surgeon gently pulls the arterial stump towards him. It is then blocked with a loop fixed to the operative fields, to diminish the heart-induced movements.
The two arterial stumps are cut to achieve equivalent diameters on both sides. The surgeon must be careful to avoid any excessive length of the artery, which can lead to later obstructive plication of the anastomosis.
We perform an end-to-end suture with a non-absorbable suture of 5.0, run in a similar fashion as the bronchial anastomosis. The posterior suture is performed first with a double-needle suture, from the top to the bottom. Both sides of the suture are then used to perform running stitches for the anterior wall. The two halves of the suture are left on a clamp, and they will be tied together after the flush has been done (Figure 13).

Atrial anastomosis (Figure 14)
The atrial anastomosis is the last one we perform. A clamp with a 120° angle is placed as far as possible in the pericardial space, while taking care not to injure a coronary artery, or alter the hemodynamic function. The clamp, as for the PA, can be stabilized with a stitch fixed on the operative field.
The stumps of the veins are pulled with atraumatic clamps, and are then opened by resection of the stapler lines. The two openings are joined after connection has been made with a dissector, creating one unique opening of the left atrium and allowing the surgeon to perform a single anastomosis.
We use the same technique as for the PA with an end-to-end anastomosis, performed by a running suture with a single non absorbable, double needled 4.0 stitch. Then, both surgical and the anesthesiological teams prepare for lung reperfusion (Figure 15).

Perfusion and ventilation in the new lung
For the transplanted lung to be functional, three points must be checked:
- De-airing the vascular bed of the lung to avoid cerebral or coronary embolisms;
- Performing a progressive artery declamping in order to achieve a low pressure lung reperfusion;
- Performing a protective lung reventilation: FiO2 of 40%, a tidal volume of 5 to 7 mL/kg of donor body weight, and a PEEP of 5 cmH2O.
A retrograde and then anterograde flush is performed. Removing the atrial clamp insures a low pressure lung reperfusion. Progressive PA declamping also aims to prevent pulmonary graft dysfunction resulting from reperfusion edema.
Retrograde flush
The flush is performed from the atrial anastomosis, with a low pressure (15 mmHg), towards the PA. The atrial clamp is slowly opened while the arterial one is left in place to prevent massive hemorrhage in case of a misfit anastomosis. Gentle ventilation helps the flush: volumes of 5 mL/kg, positive expiratory pressure of 5 cmH2O, FiO2 of 40%. When the vascular structures are filled, the retrograde de-airing is performed through the arterial suture, by opening the anterior wall of the anastomosis (Figure 16).

The atrial clamp is then closed again and an anterograde flush by opening the arterial clamp is performed. The arterial suture is tied while the flush continues at the atrial anastomosis. After a few minutes the atrial suture is tied as well. The lung is functioning again, with as protective a ventilation as possible (low FiO2 and volume ventilation).
Anterograde flush
The arterial clamp is opened first while the atrial clamp is kept closed. This flush is performed with a high pressure (except under ECMO), meaning that the arterial clamp should be opened slowly and carefully. The flush is performed first at the arterial suture, the vascular structures are filling up, and the atrial anastomosis is finally flushed. Gentle ventilation of the lung is given as previously described.
The arterial clamp is closed, and a retrograde flush is performed by opening the atrial clamp. The suture is tied, the atrial clamp is left opened, and the arterial clamp is slowly opened.
In case of CBP, the pump flow must be decreased during the flush to allow a minimal pressure in the PA.
The lung is also rewarmed using hot saline solution to wash the pleural space. The surgical and anesthesiological teams wait for approximately 20 minutes to assess the function of the new lung (hemodynamic status, blood gas) before the other side is started. While waiting, the surgeon checks for any potential sources of bleeding, haemostatic patches can be placed on the anastomoses, and chest tubes (one apical, and one posterobasal) are placed in the chest cavity. The chest wall is not closed until the other side is finished.
The same technique is used for the second side. Due to anatomical features however, hemodynamic instability may occur during the operation on the left side, especially because of the necessity of retracting the heart to facilitate exposure of the left hilum in the rather small chest cavities of some recipients. In this situation, when the operative approach consists of a bilateral anterolateral thoracotomy, the sternum should be divided transversally and the pericardium opened anteriorly to expose the heart. The heart is then lifted out of the pericardial sac and maintained gently in that position. This maneuver generally improves hemodynamics while exposing the left hilum adequately for graft implantation. Nevertheless, intraoperative ECMO may become necessary due to insufficient oxygenation on single-lung ventilation, hemodynamic instability, or the onset of reperfusion edema in the first implanted lung, especially in patients with secondary pulmonary arterial hypertension. Indeed, clamping of the artery is the crucial point of the second half of the transplant, as the newly implanted lung will receive full cardiac output and will have to provide the entire gas exchange. This is often the point where cardiopulmonary support is needed, if it has not been used before.
Usual criteria for ECMO, whether during the first lung implantation or the second one, are:
- Decrease of the cardiac index <1.5 L/min;
- Median pulmonary arterial pressure over 40–50 mmHg despite optimal nitric oxide therapy;
- SaO2 <90% or major respiratory acidosis with a FiO2 of 100% and optimal ventilation;
- Dysfunction of the right ventricle: hypokinesis, dilatation, paradoxical septum on echocardiography.
Thoracotomy closure
At the end of the procedure, after placement of at least two chest tubes in each pleural cavity, both thoracotomies are closed in a similar way as usual thoracic procedures. If the sternum has been opened, it is closed with separated stitches of steel suture. We also usually use an X steel point including the sternum and the intercostal space or only the intercostal space in its mediastinal part to prevent sternal pseudoarthrosis.
At this point, the patient can become hemodynamically instable, the chest wall causing cardiac tamponade, especially when reperfusion edema has occurred. It may be necessary for the surgeon to perform lung volume reduction to allow definitive closure. Wedge resections “on demand” are usually performed. However, on the right side, a middle lobectomy can be safely done, while on the left side resection of the lingula may be performed as well. If volume reduction is not sufficient, the chest wall can be left opened with a delayed closure until the edema has resolved.
The anesthesiologist changes the double-lumen tube for a conventional single-lumen tube before the patient is transferred to the intensive care unit.
The indications and management of post-operative ECMO are addressed elsewhere.
Technical problems and management
Vascular stenosis
Stenosis is the main complication of vascular sutures. Five mechanisms can be involved and should addressed:
- Transluminal stitch through both walls of the suture;
- Excessive tying of the running suture performed on a low pressure vessel;
- Inadequate positioning of the two stumps leading to a torsion of the anastomosis;
- Excessive length of vessel and a subsequent obstructive plication;
- External compression of the vessel by excess of the peribronchial tissue wrapping the bronchial anastomosis.
Vascular injury
The veins or the artery can also be injured during the procurement.
For the artery, if the wound is proximal, it will be removed while preparing the stump and will have no consequences, provided that a sufficient length of vessel is available at the level of the recipient artery. If the wound is distal, direct suture with non-absorbable 5.0 suture should be performed, and additional patch repair using remaining donor vessels can also be useful.
For the veins, when the inferior vein has been injured while dividing the pulmonary ligament, careful reconstruction by direct suture can be performed.
If the injury is more important, for examples when the ostia are involved or if the cuff is too short, reconstruction with pericardial tissue can be performed (23-25) (Figure 17).
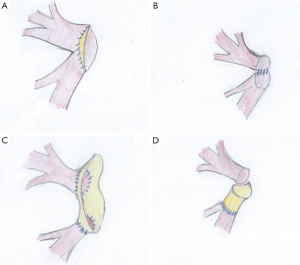
Anatomical variations
The upper right lobe bronchus in the donor lung can originate directly from the trachea. If it is a segmentary bronchus, it can be sacrificed and the collateral airway structures will be enough for ventilation of the upper lobe. If the whole upper right lobe bronchus is involved a lobectomy can be performed, or the bronchus can be sutured to the truncus intermedius (26). Another alternative would be to perform left lung transplantation only.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary lung fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Patterson GA, Cooper JD, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg 1988;45:626-33. [Crossref] [PubMed]
- Patterson GA, Todd TR, Cooper JD, et al. Airway complications after double lung transplantation. J Thorac Cardiovasc Surg 1990;99:14-20. [PubMed]
- Bisson A, Bonnette P. A new technique for double lung transplantation. “Bilateral single lung transplantation J Thorac Cardiovasc Surg 1992;103:40-6. [PubMed]
- Pasque MK, Cooper JD, Kaiser LR, et al. Improved technique for bilateral lung transplantation:rationale and clinical experience. Ann Thorac Surg 1990;49:785-91. [Crossref] [PubMed]
- Kaiser LR, Pasque MK, Trulock EP, et al. Bilateral sequential lung transplantation:the procedure of choice for double lung replacement. Ann Thorac Surg 1991;52:438-45. [Crossref] [PubMed]
- Fischer S, Strüber M, Simon AR, et al. Video-assisted minimally invasive approach in clinical bilateral lung transplantation. J Thorac Cardiovasc Surg 2001;122:1196-98. [Crossref] [PubMed]
- Gust L, D'Journo XB, Brioude G, et al. Lung dissection. Asvide 2018;5:432. Available online: http://www.asvide.com/article/view/24494
- Gust L, D'Journo XB, Brioude G, et al. Preparation of the graft. Asvide 2018;5:433. Available online: http://www.asvide.com/article/view/24495
- Gust L, D'Journo XB, Brioude G, et al. Explantation of the native lung. Asvide 2018;5:434. Available online: http://www.asvide.com/article/view/24496
- Gust L, D'Journo XB, Brioude G, et al. Preparation of the hilum. Asvide 2018;5:435. Available online: http://www.asvide.com/article/view/24497
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Parekh K, Patterson A. Technical considerations in adult lung transplantation. Semin Thorac Cardiovasc Surg 2004;16:322-32. [Crossref] [PubMed]
- Aigner C, Jaksch P, Seebacher G, et al. Single running suture--the new standard technique for bronchial anastomoses in lung transplantation. Eur J Cardiothorac Surg 2003;23:488-93. [Crossref] [PubMed]
- Garfein ES, Ginsberg ME, Gorenstein L, et al. Superiority of end-to-end versus telescoped bronchial anastomosis in single lung transplantation for pulmonary emphysema. J Thorac Cardiovasc Surg 2001;121:149-154. [Crossref] [PubMed]
- Garfein ES, McGregor CC, Galantowicz ME, et al. Deleterious effects of telescoped bronchial anastomosis in single and bilateral lung transplantation. Ann Transplant 2000;5:5-11. [PubMed]
- Gust L, D'Journo XB, Brioude G, et al. Bronchial anastomosis. Asvide 2018;5:436. Available online: http://www.asvide.com/article/view/24498
- Daly RC, McGregor CGA. Routine immediate direct bronchial artery revascularisation for single lung transplantation. Ann Thorac Surg 1994;57:1446-52. [Crossref] [PubMed]
- Yacoub M, Al-Kattan KM, Tadjkarimi S, et al. Medium term results of direct bronchial artery revascularisation using IMA for single lung transplantation (SLT with direct revascularisation). Eur J Cardiothorac Surg 1997;11:1030-6. [Crossref] [PubMed]
- Gust L, D'Journo XB, Brioude G, et al. Arterial anastomosis. Asvide 2018;5:437. Available online: http://www.asvide.com/article/view/24499
- Gust L, D'Journo XB, Brioude G, et al. Atrial anastomosis. Asvide 2018;5:438. Available online: http://www.asvide.com/article/view/24500
- Gust L, D'Journo XB, Brioude G, et al. Retrograde flush. Asvide 2018;5:439. Available online: http://www.asvide.com/article/view/24501
- Casula RP, Stoica SC, Wallwork J, et al. Pulmonary vein augmentation for single lung transplantation. Ann Thorac Surg 2001;71:1373-4. [Crossref] [PubMed]
- Oto T, Rabinov M, Negri J, et al. Techniques of reconstruction for inadequate donor left atrial cuff in lung transplantation. Ann Thorac Surg 2006;81:1199-204. [Crossref] [PubMed]
- D'Journo XB, Gariboldi V, Trousse D, et al. Techniques de transplantation bipulmonaires. EMC - Techniques chirurgicales - Thorax 2011:1-15 [Article 42-440-C].
- Schmidt F, McGiffin DC, Zorn G, et al. Management of congenital abnormalities of the donor lung. Ann Thorac Surg 2001;72:935-7. [Crossref] [PubMed]