Combined thoracoscopic and laparoscopic minimally invasive esophagectomy
Introduction
Esophagectomy remains effective for patients with localized esophageal carcinoma (1). However, invasive esophageal cancer surgery carries a high incidence of complications, and serious complications still occur although improvements have been made in surgical maneuvers and perioperative care. Regardless of the approach, open esophagectomy has been associated with considerable morbidity and mortality, the incidence of major or minor complications is still 70-80%, and the operative mortality is 4-7% in experienced surgery centers (2). Great progress has been made in video-assisted thoracoscopic technique during the past 20 years. Since thoracoscopic surgery involves minimal intercostal incisions, respiratory function can be retained, so the pulmonary complications such as pneumonia and atelectasis decrease (3-5). Many esophageal cancer surgeries take a long time, bringing stress to the operating staffs as well as the patients, so it’s urgent to improve surgical procedures and reduce the operation duration (6,7). As the experience and skills for thoracoscopic and laparoscopic surgery improve, thoracoscopic and laparoscopic esophagectomy (TLE) has attracted increasing attention and becomes an alternative to open three-field esophagectomy with fewer complications.
The technique of VATS esophagectomy described here is currently employed in the department of thoracic surgery at Sichuan Provincial People’s Hospital of China (Video 1).
Clinical summary
A 59-year-old lady presented with progressive dysphagia for three months. Gastroscopy revealed a nodular neoplasm in the esophagus 25-30 cm away from the incisor teeth. Pathological biopsy demonstrated esophageal squamous cell carcinoma. CT scan revealed the middle of the esophageal wall was thickening and mediastinal lymph nodes were enlarged.
Formal spirometry showed a FEV1 of 3.02 (109.3% predicted), a FVC of 3.80 (105% predicted) and an FEV1/FVC ratio of 82.49%.
Pre-operative assessment
Esophageal cancer surgery is associated with a high incidence of complications, especially the anastomotic leakage and pulmonary complication. Therefore pre-operative selection and assessment of patient may be crucial. Moreover, the location and size of the tumor, foreign invasion, lymph node metastasis, heart and lung function are to be evaluated before operation. Operator is also a key factor and individuals unfamiliar with VATS techniques should not attempt VATS esophagectomy.
Anaesthesia and positioning
General anesthesia was performed with double lumen intubation, which allowed independent ventilation of either lung for better exposure. Meanwhile, artificial pneumothorax was employed and the patient was kept in left lateral position leaning forward with upper limb suspended, in which way, the posterior mediastinal tissues were better exposed.
Technique
Four VATS ports were made in right chest wall for optimal views of the posterior hilum and instrument insertion: one incision about 5 mm in length in the third intercostal axillary line and fourth intercostal anterior axillary as the operation port, and one incision 12 mm in length in the seventh intercostal axillary midline and eighth intercostal axillary line as the operating hole and the observation hole; cervical and abdominal incision include: incision over the front of left sternocleidomastoid muscle and 5-12 mm incision over subxiphoid, navel, left and right midclavicular line subcostal (Figure 1).
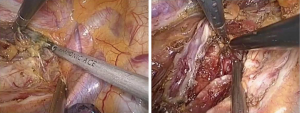
The first step in the procedure is to confirm resectability and to identify invasion of the chest wall, pleurae and hilar structures including the aorta, pulmonary artery and bronchus. The second step is thoracic operation: performing anatomical esophageal resection completely, the principle and the extent of resection of which is similar to conventional open thoractomy. Electric coagulation hook and ultrasonic knife were used in esophageal isolation from top to bottom, clamp the two ends of arch of azygos vein and cut them off with Hemolok. All lymph nodes in the operative field were dissected, including lymph nodes of paraesophageal, carina, left and right recurrent laryngeal nerve chains (Figure 2). Intraoperative care was required to avoid inadvertent injury to the vagus, phrenic, recurrent laryngeal nerves and membranous part of trachea. Then, a 32 F chest drain was placed through the anterior port before closure. The third step is gastric dissociation: assisted by laparoscopy, ultrasonic scalpel dissected gastric greater and lesser curvature, fully separated the left gastric artery and vein, and then clamped the two ends of left gastric artery and vein to cut them off with Hemolok. Stomach was fully separated with attentions paid to avoid injury of adjacent vessels, spleen and liver. The last step we dissected cervical esophagus, made a pipe type gastric (Figure 3), a circular stapler was used to complete cervical esophagogastrostomy. Operation time: 210 min, intraoperative blood loss: 200 mL.
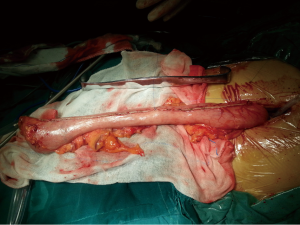
Final pathology revealed a T3N1M0 highly differentiated squamous cell carcinoma involving the vagus nerve.
Post-operative management
All patients fasted 7-9 days after operation, and underwent enteral feeding by the duodenal feeding tube from the next day after the operation. On the ninth day after operation, patient took liquid diets without exception, and chest X-ray was normal, and the chest tube was extubated.
Comments
Combined thoracoscopic and laparoscopic minimally invasive esophagectomy is a safe and reliable option for esophageal carcinoma with smaller trauma, shorter hospital stay, less blood loss, less pain and better appearance, especially for the elderly and those with poor lung function. As reported by Richards, this approach also improves the view of the mediastinal node packets which facilitates lymphadenectomy (8). TLE is technically complex, so a steep learning curve is required to reach optimal surgical outcomes, however, thoracic surgeons will have to treat more cases to climb the learning curve because most of them are not familiar with laparoscopic skills (9).
Acknowledgements
All authors have the same contribution to this article.
Disclosure: The authors declare no conflict of interest.
References
- Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23. [PubMed]
- Pierre AF, Luketich JD. Technique and role of minimally invasive esophagectomy for premalignant and malignant diseases of the esophagus. Surg Oncol Clin N Am 2002;11:337-50. [PubMed]
- Lee JM, Cheng JW, Lin MT, et al. Is there any benefit to incorporating a laparoscopic procedure into minimally invasive esophagectomy? The impact on perioperative results in patients with esophageal cancer. World J Surg 2011;35:790-7. [PubMed]
- Parker M, Pfluke JM, Shaddix KK, et al. Video: transcervical videoscopic esophageal dissection in minimally invasive esophagectomy. Surg Endosc 2011;25:941-2. [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [PubMed]
- Suzuki S, Morimatsu H, Omori E, et al. Responses to surgical stress after esophagectomy: Gene expression of heat shock protein 70, toll-like receptor 4, tumor necrosis factor-α and inducible nitric oxide synthase. Mol Med Rep 2010;3:765-9. [PubMed]
- Udagawa H, Ueno M, Kinoshita Y. Rationale for video-assisted radical esophagectomy. Gen Thorac Cardiovasc Surg 2009;57:127-31. [PubMed]
- Carnochan FM, Walker WS. Positron emission tomography may underestimate the extent of thoracic disease in lung cancer patients. Eur J Cardiothorac Surg 2009;35:781-4; discussion 784-5. [PubMed]
- Avendano CE, Flume PA, Silvestri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg 2002;73:922-6. [PubMed]