Metastasis-associated lung adenocarcinoma transcript 1 regulates tumor progression: old wine in a new bottle
In the post-genomic era, non-coding RNAs (ncRNAs) have aroused great attention in chemistry and life sciences (1-3). Generally, ncRNAs are divided into two categories depended on the length of a transcript. Small ncRNAs, which are less than 200 nucleotides (nt) in length, include microRNA (miRNA), PIWI-interacting RNA (piRNA), promoter-associated RNA (paRNA), and small nucleolar RNA (snoRNA). Long ncRNAs (lncRNAs), which exceed 200 nucleotides, include pseudogene RNA (4,5). lncRNAs are the most numerous of the ncRNAs. lncRNAs have been comprehensively studied concerning structure and functions. lncRNAs have a transcribing length of 200–100,000 nt and lack a complete functional open reading frame (ORF). As an RNA or functional short peptide, lncRNAs play essential roles in maintaining cell differentiation, proliferation and apoptosis, as well as other functions. The disrupted expression of lncRNAs has been associated with human diseases that include diabetes mellitus (6), nervous system diseases (7), coronary artery disease (8), and cancer (1). Recent studies have implicated lncRNAs as being very important in cancer, with correlations demonstrated with cancer progression and development due to the regulation of a series of cellular signalling pathways, including the nuclear factor-kappa B pathway (9), Hippo pathway (10), and Wnt pathway (11). A series of cancer-associated lncRNAs include PVT1 (12), ANRIL (13), and TUG1 (14). lncRNAs exhibit strong tissue- and cell-specific expression patterns in humans (15), and thus are often used as tumour biomarkers for the early diagnosis and monitoring of cancer progression.
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), also known as nuclear-enriched abundant transcript 2 (NEAT2), has been the subject of many disease-associated studies. Exemplifying this, a literature searches of the PubMed database using the search term “MALAT1” returned 450 research papers (excluding editorials, meta-analyses, reviews, letters, comments, and case reports) through February 25, 2018. The numbers of research articles have increased markedly since 2013, attesting to the burgeoning focus on lncRNAs. MALAT1 has a length of approximately 8,000 nt. It was first identified as a prognostic marker for the early stage of lung adenocarcinoma (16). Studies have associated MALAT1 with tumour progression and metastasis (17,18). As research continues, increasing numbers of studies have reported the elevated expression of MALAT1 in a wide variety of cancers, including oral squamous cell carcinoma (19), breast cancer (20), and hepatocellular carcinoma (21). MALAT1 has been implicated with radio- or chemotherapy resistance and poor prognoses in patients (22-24). Altered levels of MALAT1 in animal tumour models or multiple cancer cell lines have been demonstrated to affect tumour growth, differentiation, invasion, and metastasis via a series of regulatory steps. One example is the recruitment and interaction with proteins (25), where MALAT1 acts as a co-regulator or a co-repressor (26) and interacts with miRNA (27). Moreover, MALAT1 may have potential value as a therapeutic target in cancer treatment. Arun et al. demonstrated in a mouse mammary tumour virus (MMTV)-PyMT mouse mammary carcinoma model that MALAT1 antisense oligonucleotides slow tumour growth accompanied by significant differentiation into cystic tumours and a reduction in metastasis (28).
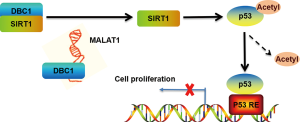
Chen et al. (29) recently reported that MALAT1 as an epigenetic regulator via its interaction with a protein termed depleted in breast cancer 1 (DBC1). The interaction regulates p53 activity and reduces the transcription of a series of its downstream target genes, which promote cell proliferation and inhibit cell apoptosis. The authors employed a high-throughput strategy, including RNA pull-down and quantitative proteomics, to screen the interacting proteins of MALAT1, and identified 127 potential MALAT1-interacting proteins. In a series of bioinformatics analyses and experimental validations, the authors verified the physical interaction between MALAT1 and amino acids 120–180 of DBC1. The same authors used mechanistic experiments to reveal that MALAT1 binding competes with the interaction between sirtuin1 (SIRT1) and DBC1, sequentially regulating the deacetylation of p53, which forms a regulatory axis designated “MALAT1-DBC1-SIRT1-P53” to affect a spectrum of p53 downstream biological functions (Figure 1). The study and the observations are compelling and important. However, the conclusion drawn by Chen et al. should be further verified for the following reasons. Firstly, although MALAT1 is almost always retained in the nucleus (30) where it is localized within nuclear speckles (31,32), studies have reported MALAT1 interaction with miRNA in the cytoplasm (21,27,33). Therefore, the subcellular localization of MALAT1 should be verified in HepG2 cells. Secondly, the conclusion of this article was relied on the use of HepG2 cells. Thus, it is not known whether the MALAT1-DBC1-SIRT1-P53 regulatory axis is relevant in other cancer cell lines or animal models. Finally, the article lays the foundation for the biological motif of MALAT1 that is located at the 3' end of MALAT1 (nt 6,918–8,441) (34). However, it is unknown whether the nuclear retention signals of MALAT1 are also located in this region. If so, the nuclear retention signals may be affected in a competitive manner by the binding of MALAT1 with the aa120–280 region of DBC1.
In summary, the findings of Chen et al. have expanded our knowledge of the MALAT1 protein interactome and have clearly established the novel regulatory network between MALAT1 and p53 in tumorigenesis, which may provide a potential strategy for the targeting therapy of MALAT1 in specific cancer patients (29). However, validation of the in vitro findings using in vivo models is necessary before clinical applications can be contemplated. With the development of next-generation sequencing technologies, more lncRNAs are being discovered and identified. Because of their unique structure, lncRNAs are very stable in disease-related tissues, cells, and serum. Furthermore, compared with protein detection, the extraction and detection of lncRNAs is being done with greater specificity, sensitivity, and stability (35). However, lncRNAs are expressed at low levels relative to miRNAs, which is a challenge to the potentially use of lncRNAs as the biomarkers for cancer diagnosis. Identification and verification of novel functional lncRNAs in vivo and in vitro is a pressing priority for the exploration of the underlying molecular mechanisms of diseases. Even though the weight of evidence clearly supports the potential value of lncRNAs for cancer diagnosis and therapeutics, these clinical applications remain elusive (36,37). We believe that novel approaches and techniques will ultimately shed light on these processes and provide a new strategy for the early screening and therapy of cancers.
Acknowledgements
Funding: This work was supported by the student innovation project of Central south university (2017zzts010), and the Hunan Provincial scientific research project of traditional Chinese Medicine (201798).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sun Q, Hao Q, Prasanth KV. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet 2017. [Epub ahead of print].
- Zhang J, Zhu Y, Wang R. Long noncoding RNAs in respiratory diseases. Histol Histopathol 2018. [Epub ahead of print]. [PubMed]
- Ou C, Sun Z, Li X, et al. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett 2017;399:53-63. [Crossref] [PubMed]
- Weng M, Wu D, Yang C, et al. Noncoding RNAs in the development, diagnosis, and prognosis of colorectal cancer. Transl Res 2017;181:108-20. [Crossref] [PubMed]
- Sana J, Faltejskova P, Svoboda M, et al. Novel classes of noncoding rnas and cancer. J Transl Med 2012;10:103. [Crossref] [PubMed]
- He X, Ou C, Xiao Y, et al. LncRNAs: key players and novel insights into diabetes mellitus. Oncotarget 2017;8:71325-41. [PubMed]
- Quan Z, Zheng D, Qing H. Regulatory Roles of Long Non-Coding RNAs in the Central Nervous System and Associated Neurodegenerative Diseases. Front Cell Neurosci 2017;11:175. [Crossref] [PubMed]
- Wu G, Cai J, Han Y, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 2014;130:1452-65. [Crossref] [PubMed]
- Ou C, Sun Z, Zhang H, et al. SPLUNC1 reduces the inflammatory response of nasopharyngeal carcinoma cells infected with the EB virus by inhibiting the TLR9/NF-kappaB pathway. Oncol Rep 2015;33:2779-88. [Crossref] [PubMed]
- Ou C, Sun Z, Li S, et al. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget 2017;8:75727-41. [Crossref] [PubMed]
- Ou C, Li X, Li G, et al. WWC3: the bridge linking Hippo and Wnt pathways in lung cancer. J Thorac Dis 2017;9:2315-6. [Crossref] [PubMed]
- Liu E, Liu Z, Zhou Y, et al. Overexpression of long non-coding RNA PVT1 in ovarian cancer cells promotes cisplatin resistance by regulating apoptotic pathways. Int J Clin Exp Med 2015;8:20565-72. [PubMed]
- Lan WG, Xu DH, Xu C, et al. Silencing of long non-coding RNA ANRIL inhibits the development of multidrug resistance in gastric cancer cells. Oncol Rep 2016;36:263-70. [Crossref] [PubMed]
- Ou C, Li G. Long non-coding RNA TUG1: a novel therapeutic target in small cell lung cancer. J Thorac Dis 2017;9:E644-5. [Crossref] [PubMed]
- Rios-Barrera LD, Gutiérrez-Pérez I, Domínguez M, et al. acal is a long non-coding RNA in JNK signaling in epithelial shape changes during drosophila dorsal closure. PLoS Genet 2015;11:e1004927. [Crossref] [PubMed]
- Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003;22:8031-41. [Crossref] [PubMed]
- Gutschner T, Hämmerle M, Diederichs S. MALAT1 -- a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl) 2013;91:791-801. [Crossref] [PubMed]
- Qiu MT, Hu JW, Yin R, et al. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol 2013;34:613-20. [Crossref] [PubMed]
- Chang SM, Hu WW. Long non-coding RNA MALAT1 promotes oral squamous cell carcinoma development via microRNA-125b/STAT3 axis. J Cell Physiol 2018;233:3384-96. [Crossref] [PubMed]
- Zhang P, Zhou H, Lu K, et al. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. Onco Targets Ther 2018;11:291-9. [Crossref] [PubMed]
- Chen L, Yao H, Wang K, et al. Long Non-Coding RNA MALAT1 Regulates ZEB1 Expression by Sponging miR-143-3p and Promotes Hepatocellular Carcinoma Progression. J Cell Biochem 2017;118:4836-43. [Crossref] [PubMed]
- Chen W, Xu XK, Li J L, et al. MALAT1 is a prognostic factor in glioblastoma multiforme and induces chemoresistance to temozolomide through suppressing miR-203 and promoting thymidylate synthase expression. Oncotarget 2017;8:22783-99. [PubMed]
- Jadaliha M, Zong X, Malakar P, et al. Functional and prognostic significance of long non-coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget 2016;7:40418-36. [Crossref] [PubMed]
- Lu H, He Y, Lin L, et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol 2016;37:1683-91. [Crossref] [PubMed]
- Ji Q, Zhang L, Liu X, et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer 2014;111:736-48. [Crossref] [PubMed]
- Hirata H, Hinoda Y, Shahryari V, et al. Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205. Cancer Res 2015;75:1322-31. [Crossref] [PubMed]
- Xiao X, Zhou T, Guo S, et al. LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol 2017;243:404-12. [Crossref] [PubMed]
- Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev 2016;30:34-51. [Crossref] [PubMed]
- Chen R, Liu Y, Zhuang H, et al. Quantitative proteomics reveals that long non-coding RNA MALAT1 interacts with DBC1 to regulate p53 acetylation. Nucleic Acids Res 2017;45:9947-59. [Crossref] [PubMed]
- Miyagawa R, Tano K, Mizuno R, et al. Identification of cis- and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA 2012;18:738-51. [Crossref] [PubMed]
- Hutchinson JN, Ensminger AW, Clemson CM, et al. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 2007;8:39. [Crossref] [PubMed]
- Bernard D, Prasanth KV, Tripathi V, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J 2010;29:3082-93. [Crossref] [PubMed]
- Chen H, Wang X, Yan X, et al. LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFkappaB. Int Immunopharmacol 2018;55:69-76. [Crossref] [PubMed]
- Xu C, Yang M, Tian J, et al. MALAT-1: a long non-coding RNA and its important 3' end functional motif in colorectal cancer metastasis. Int J Oncol 2011;39:169-75. [PubMed]
- Ou C, Li G. Exosome-transmitted lncARSR: a novel therapeutic target in renal cancer. Transl Cancer Res 2017;6:656-7. [Crossref]
- He X, Kuang G, Ou C, et al. Crosstalk between circular RNAs and microRNAs in tumorigenesis. Transl Cancer Res 2017;6:S1448-50. [Crossref]
- He X, Ou C. Exosome-derived microRNAs in cancer progression: angel or devil?. J Thorac Dis 2017;9:3440-2. [Crossref] [PubMed]