Novel biologic factors correlated to visceral pleural invasion in early-stage non-small cell lung cancer less than 3 cm
Introduction
Lung cancer is the leading cause of cancer related death worldwide (1,2), and non-small cell lung cancer (NSCLC) is reported to account for about 85% of all lung cancers. With the advancement of medical screening methods, more and more cases of early-stage NSCLC are being discovered (3). As for the prognosis of early-stage NSCLC, besides tumor size and lymph node metastasis, pleural invasion is also found to be a significant prognostic factor (4,5). Previous studies have shown that patients with visceral pleural invasion (VPI) yield significantly worse survival than patients without (5,6). In the 8th edition of the TNM classification for NSCLC, if a tumor shows VPI, it increases the T descriptor from T1 to T2 and upstages a tumor from stage IA to stage IB, even when the tumor is less than 3 cm in size (7). Traditionally, the underline mechanism of VPI is instinctively believed to be the result of too much close distance between cancerous lesion and the visceral pleura. However, this explanation cannot fully answer the question why the postoperative prognosis of NSCLCs less than 3 cm with positive VPI is still worse than that of those without, considering the fact that the physical factor of distance had already disappeared after the tumor was completely resected. Therefore, there must be some uncovered biologic factors, beyond that physical factor, affecting long-term prognosis. As a result, it is of great importance to investigate further on the clinicopathological characteristics of VPI and factors correlated to VPI in NSCLC, especially in early-stage NSCLC (diameter ≤3 cm) comprehensively. Previously, only a scarce of studies have explored the correlated factors of VPI in NSCLC and controversial conclusions have been made among these studies (8-10). Moreover, most of these studies mainly focused on computed tomography (CT) characteristics without analysing the impact of pathological features on VPI (8-10). Therefore, in this study, we tried to investigate comprehensively on the factors correlated closely to VPI in early-stage NSCLC by analysing both clinical and pathological characteristics. To our knowledge, our study is the most comprehensive analysis of correlated factors of VPI in early-stage NSCLC with the largest sample size.
Methods
Patients
We retrospectively collected the clinicopathological data of patients who underwent lobectomy or segmentectomy with systematic lymph node dissection or lymph node sampling for NSCLC at our department between January 2015 to December 2016. Contrast-enhanced chest CT, brain magnetic resonance imaging or CT, upper abdominal CT, bone scanning, and cardiopulmonary tests were routinely performed in all patients before surgery. Inclusion criteria included: (I) cancer nodule ≤3 cm in diameter measured on chest CT scans without nodal and distant metastases; (II) patients received lobectomy or segmentectomy with systematic lymph node dissection or lymph node sampling. The following patients were excluded from the study: (I) patients who had received chemotherapy, radiotherapy and/or chemoradiotherapy preoperatively; (II) patients with synchronous multiple primary lung cancers or secondary pulmonary cancers. Since our study was a retrospective study and analyzed anonymously, the committee waived the need for consent.
Preoperative clinical data on age, gender, tumor history, tumor location, tumor size and distance to visceral pleura as well as tumor features including pleural indentation [defined as tumor indentation of the visceral pleura connecting the tumor to the pleura (10,11)] and tumor margin spiculation on CT scans were collected. Postoperative pathological data on tumor type, differentiation, cancer embolus, lymph node involvement and VPI were also well collected. According to the definition of VPI for TNM staging system (4), VPI was categorized into three groups: PL0 as no pleural involvement; PL1 as invasion beyond the elastic layer without being exposed on the pleural surface; PL2 as invasion to the surface of the visceral pleura. PL0 was defined as without VPI, while PL1 and PL2 were both defined as VPI (4). Because we aimed to explore the correlated factors of VPI in early-stage NSCLC, we simply divided those patients into two groups according to status of VPI (VPI negative group and VPI positive group).
Statistical analysis
Data were represented as the mean ± standard deviation (SD) for continuous variables or number and percentage (%) for categorical variables. Student’s t-test or the Mann-Whitney non-parametric U-test was applied for comparing continuously distributed data between groups, and Chi-squared test or Fisher’s exact test was applied to categorical data between groups. Multivariate logistic regression analysis was applied to identify significantly correlated factors for VPI. The statistical analysis was performed using the SPSS 22.0 (IBM, Armonk, NY, USA). A two-sided P value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the included patients with early-stage NSCLC
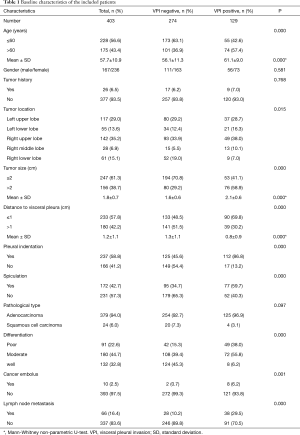
A total of 403 patients who met the inclusion criteria were included for analysis. The baseline characteristics of those patients were shown in Table 1. The mean age of those patients was 57.7±10.9 years old with a male to female ratio of 1:0.7. Only a small proportion of patients (6.5%) had previous tumor history. Most of those NSCLCs were located in left and right upper lobes. The mean size of those NSCLCs was 1.8 cm, and the mean distance between the cancers to visceral pleura was 1.2 cm. On CT scans, more than half of those NSCLCs (58.8%) showed pleural indentation, and nearly half of those NSCLCs (42.7%) had spiculation. Postoperatively, most of those NSCLCs (94.0%) were found to be adenocarcinomas, and the rest were squamous cell carcinomas (6.0%). About one third of those NSCLCs (32.8%) were found to be well differentiated, and 44.7% of them were moderately differentiated, and the rest (22.6%) were poorly differentiated. Only a small proportion of those NSCLCs (2.5%) were found to have cancer embolus within the cancer tissues. Sixty-six of those NSCLCs (16.4%) were found to have positive lymph node metastasis. As for the status of VPI, 129 patients (32.0%) were found to have VPI, while 274 patients (68.0%) have negative VPI.
Full table
Baseline characteristics of patients with positive VPI
A total of 129 patients were found to have positive VPI pathologically. The baseline characteristics of patients with positive VPI were shown in Table 1. The mean age of those patients was 61.1±9.0 years old with a male to female ratio of 1:0.8. Only a small proportion of patients (6.5%) had previous tumor history. Most of those NSCLCs with positive VPI were located in left and right upper lobes. The mean size of those NSCLCs with positive VPI was 2.1 cm, and the mean distance of the cancers to visceral pleura was 0.8 cm. On CT scans, most of those NSCLCs with positive VPI (86.8%) showed pleural indentation, and more than half of those NSCLCs with positive VPI (59.7%) had spiculation. Postoperatively, most of those NSCLCs with positive VPI (96.9%) were found to be adenocarcinomas. Only a small proportion of those NSCLCs with positive VPI (6.2%) were found to be well differentiated, and more than half of them (55.8%) were moderately differentiated, and more than one third of them (38.0%) were poorly differentiated. Only a small proportion of those NSCLCs with positive VPI (6.2%) were found to have cancer embolus within the cancer tissues. Thirty-eight of those early-stage NSCLCs with positive VPI (29.5%) were found to have positive lymph node metastasis.
Comparison of characteristics between patients with VPI and patients without
The characteristics of patients with VPI were compared with those without VPI in Table 1. Patients with VPI had significantly older age than those without (61.1 vs. 56.1 years; P<0.001). When those patients were grouped into two groups by age, the percentage of patients with an age of >60 years in the VPI positive group was significantly higher than that in VPI negative group (57.4% vs. 36.9%; P<0.001). There was no significant difference of gender ratio and rate of previous tumor history between those two groups. As for tumor location, VPI was more likely to be involved in NSCLCs located in right upper and middle lobes. The mean size of NSCLCs with VPI was significantly larger than those without (2.1 vs. 1.6 cm; P<0.001), and when those patients were grouped into two groups by tumor size, the percentage of patients with a tumor size of >2 cm in the VPI positive group was significantly higher than that in VPI negative group (58.9% vs. 29.2%; P<0.001). Moreover, NSCLCs with VPI were located significant closer to visceral pleura than those without (0.8 vs. 1.3 cm; P<0.001), and when those patients were grouped into two groups by nodule’s distance to the visceral pleura, the percentage of cancerous nodules with an distance of ≤1 cm in the VPI positive group was significantly higher than that in VPI negative group (69.8% and 48.5%; P<0.001). On CT scans, NSCLCs with VPI showed significantly larger rates of pleural indentation (86.8% vs. 45.6%; P<0.001) and spiculation (59.7% vs. 34.7%; P<0.001) than those without. Postoperatively, NSCLCs with VPI tended more likely to be adenocarcinomas (96.9% vs. 92.7%; P=0.097). Notably, NSCLCs with VPI were more likely to be poorly differentiated than those without (the percentage of poorly differentiated NSCLCs: 38.0% vs. 15.3%; P<0.001). Moreover, NSCLCs with VPI were significantly more likely to have cancer embolus in the cancer tissues than those without (6.2% vs. 0.7%; P=0.001). In addition, NSCLCs with VPI were also significantly more likely to have lymph node metastasis than those without (29.5% vs. 10.2%; P<0.001).
Multivariate logistic regression analysis for identifying correlated factors for VPI
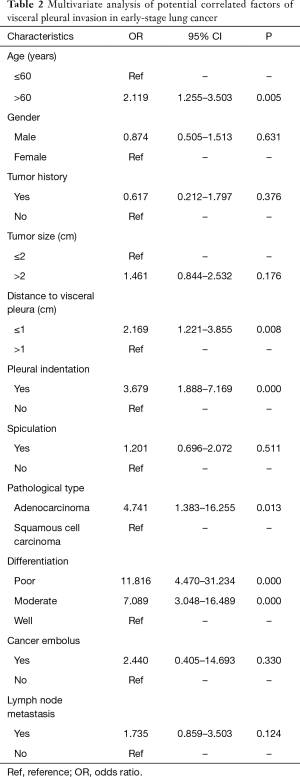
In order to identify potential factors closely correlated to VPI in early-stage NSCLC, a multivariate logistic regression analysis was performed (Table 2). Age, distance to visceral pleura, pleural indentation, pathological type, and tumor differentiation were found to be significantly correlated to VPI in early-stage NSCLC. Patients with older age [odds ratio (OR) =2.119, 95% CI: 1.255–3.503; P=0.005], shorter distance to visceral pleura (OR =2.169, 95% CI: 1.221–3.855; P=0.008), pleural indentation (OR =3.679, 95% CI: 1.888–7.169; P<0.001), adenocarcinoma (OR =4.741, 95% CI: 1.383–16.255; P=0.013), and poor tumor differentiation (OR =11.816, 95% CI: 4.470–31.234; P<0.001) were more likely to have VPI.
Full table
Discussion
Pleural invasion remains to be an important prognostic factor of NSCLC, especially in early-stage NSCLC (5,12). The 5-year survival rates of NSCLC patients were reported to decrease from 75.9–80% (for patients without VPI) to 54.1–63.6% (for patients with VPI) (13,14). Instinctively, the underline mechanism of VPI is believed to be the result of too much close distance between cancerous lesion and the visceral pleura. However, this explanation cannot fully answer the question why the postoperative prognosis in NSCLCs ≤3 cm is still worse in patients with VPI than in those without, considering that the physical factor of distance had already disappeared after the tumor was completely resected. There must be some uncovered biologic factors, beyond physical factor, affecting long-term prognosis. Previous studies mainly focused on exploring how to predict VPI using preoperative clinical factors (8-10), while this study focused on exploring the biological factors correlated to VPI, which might give us a comprehensive understanding of VPI. Therefore, in our current study, in order to explain why patients with positive VPI had a poor prognosis from clinical point of view, we investigated comprehensively on the correlated factors of VPI in early-stage NSCLC by combining both clinical and pathological characteristics. In this study, a total of 403 NSCLC patients with a tumor size ≤3 cm were included for analysis, in which there were 129 patients with positive VPI. On multivariate logistic regression analysis, besides shorter distance to visceral pleura, pleural indentation, elderly, adenocarcinoma, and poor tumor differentiation were also found to have significant impact on the status of VPI in early-stage NSCLC. Therefore, elderly, pathological type of adenocarcinoma, and poor tumor differentiation were fund to be novel biologic correlated factors of VPI in early-stage NSCLC.
VPI has been regarded as an independent indicator of lung cancer invasiveness and aggressiveness (10,15). In pathology, we found that NSCLCs with VPI were more likely to be poor differentiated than those without (P<0.001). Previous study has also observed similar results that there were significantly more tumors with VPI in patients with a tumor of moderate or poor differentiation (P<0.001) (15). Zhang et al. (16) found that more lung cancers with VPI tended to be poorly differentiated compared to those without (30.1% vs. 25.9%; P=0.159). Moreover, we found that poor tumor differentiation was also significantly correlated to VPI in early-stage lung cancer (poor differentiation: OR =11.816, 95% CI: 4.470–31.234; P<0.001; moderate differentiation: OR =7.089, 95% CI: 3.048–16.489; P<0.001) in the multivariate analysis. Because tumors with poor differentiation were significantly correlated with overexpression of unfavorable prognostic biomarkers such as Vimentin (17) and RBX1 (18), showing more aggressive and invasive biologic behavior than those well-differentiated tumors. Therefore, VPI may be similarly correlated to those unfavorable biomarkers, which may explain why patients with VPI had worse prognosis than those without. Notably, in our study, we found that the percentage of adenocarcinoma in VPI positive group tended to be higher than that in VPI negative group (96.9% vs. 92.7%; P=0.097) and in the multivariate analysis, we found that pathological type was significantly correlated to VPI in early-stage NSCLC. Previously, Lakha et al. have also found that compared to patients without VPI, patients with VPI were more likely to present with adenocarcinoma (P<0.001) (6). However, most of previous studies did not found pathological type to be a significantly correlated factor of VPI in lung cancer (8-10), which we believe was due to very limited cases of squamous cell carcinoma available for analysis. Previous experimental study has shown that lung adenocarcinoma was more aggressive and invasive than squamous cell carcinoma with a poorer prognosis (19). Therefore, our finding that adenocarcinoma is significantly correlated to VPI added to the evidence pool that adenocarcinoma has more aggressive and invasive biologic behavior from clinical analysis. Moreover, in our study, similar to previous studies (10,15,16), we found that NSCLCs with VPI were more likely to have lymph node metastasis than those without (29.5% vs. 10.2%; P<0.001), which added to the evidence that VPI served as an indicator of lung cancer invasiveness and aggressiveness thus leading to a poor prognosis. Taken together, VPI was related to poor tumor differentiation, adenocarcinoma, and lymph node metastasis, which may help explain why VPI was a significant unfavorable prognostic factor of early-stage NSCLC patients. Therefore, our results might justify providing adjuvant therapy for patients with VPI-positive early-stage NSCLC.
Interestingly, in our study, patients with VPI showed significantly older mean age than patients without (61.1 vs. 56.1 years; P<0.001). Similar result has also been observed by Lakha et al. (6), and they found that the percentage of patients more than 60 years old in VPI positive group was significantly higher than that in VPI negative group (P=0.02). Moreover, Ebara et al. found that patients with VPI tended to be older than patients without (68.5 vs. 65.9 years; P=0.061) (8). However, other studies did not find any difference of age between patients with VPI and those without (9,10,15,16,20,21). But surprisingly, in our study, older age was found to be a significantly correlated factor of VPI in early-stage NSCLC (OR =2.119, 95% CI: 1.255–3.503; P=0.005) in the multivariate analysis. However, previous evidence has shown that lung cancers in aged individuals have a less invasive behavior than those in younger ones (22). One possible reason for our result that older age was significantly correlated to VPI in early-stage NSCLC is that in China, older patients have less utilization of screening than the younger ones and often ignore slight symptoms due to the fact that they have retired. Therefore, older patients are less likely to be referred to clinic, which leads to a more advanced stage in older patients.
NSCLCs with VPI showed larger tumor size than those without (2.1 vs. 1.6cm; P<0.001) in our study. Previously, Ebara et al. (8) have also found that the mean tumor size of lung cancer with VPI was significantly larger than that of those without VPI in early-stage lung cancer (1.67 vs. 1.43 cm; P=0.001). Several other studies have also showed that VPI was found more in lung cancers with larger tumor size (6,10,16). However, Qi et al. (9), Nitadori et al. (20) and Hsu et al. (21) did not found any difference of size between lung cancers with VPI and those without. In the multivariate logistic regression analysis, tumor size was not found to be significantly correlated to VPI in our study, which agreed with most of previous studies (8,9). It is believed that tumor size does have certain impact on its invasive behavior, but in our study, all those NSCLCs had a tumor size less than 3 cm. Therefore, for those early-stage NSCLCs (cT1), tumor size may show less impact on VPI than other factors. Traditionally, VPI in lung cancer is believed to be the result of too much close distance between cancerous lesion and the visceral pleura. In our study, NSCLCs with VPI were located closer to the visceral pleura than those without (mean distance: 0.8 vs. 1.3 cm; P<0.001). Previously, Ebara et al. (8) have also shown that distance to visceral pleura was shorter in lung cancers with VPI than those without (mean distance: 1.2 vs. 1.5 cm; P=0.003). Moreover, Qi et al. (9) have also found that more lung cancers with VPI were located within 5mm to visceral pleura (P<0.001). Besides Qi et al.’s study (9), our study also proved that shorter distance to visceral pleura (OR =2.169, 95% CI: 1.221–3.855; P=0.008) was significantly correlated to VPI in early-stage NSCLC in the multivariate analysis. It is reasonable that the closer a tumor is located to the visceral pleura, the more likely it is to have VPI. On CT scan, NSCLCs with VPI were more likely to have pleural indentation than those without and pleural indentation was found to be significantly correlated to VPI in early-stage NSCLC (OR =3.679, 95% CI: 1.888–7.169; P<0.001) in the multivariate analysis. Previous studies have also proved that pleural indentation was an independent predictive factor of VPI in lung cancer (9,10). Because pleural indentation may suggest the existence of a tumor infiltrating the pleural or pleural dissemination, it serves as a sign suggestive of pulmonary malignancy and possible diagnosis of VPI (9,10,21). Therefore, our study added to the evidence that pleural indentation was a significantly correlated factor of VPI in early-stage NSCLC.
Our study has several limitations. First, our study is a retrospective study, which could limit our analytical validity. Second, in this study, we only grouped patients into VPI positive group and VPI negative group and did not analyse the correlated factors of PL1 and PL2 groups respectively. Finally, even though our study has the largest sample size compared to previous studies, more similar studies and multicenter researches are needed to update and confirm our current conclusions.
Conclusions
In this study, we found that age, distance to visceral pleura, pleural indentation, pathological type, and tumor differentiation had significantly impacts on the status of VPI in early-stage NSCLC. Therefore, besides shorter distance to visceral pleura and pleural indentation, elderly, adenocarcinoma, and poor differentiation were novel biologic factors significantly correlated to VPI in early-stage NSCLC.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81672291; No. 31071210) (to YD Lin).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of West China Hospital (No. 20171217).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [PubMed]
- Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90.
- Huang H, Wang T, Hu B, et al. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg 2015;99:1130-9. [Crossref] [PubMed]
- Lakha S, Gomez JE, Flores RM, et al. Prognostic significance of visceral pleural involvement in early-stage lung cancer. Chest 2014;146:1619-26. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Ebara K, Takashima S, Jiang B, et al. Pleural invasion by peripheral lung cancer: prediction with three-dimensional CT. Acad Radiol 2015;22:310-9. [Crossref] [PubMed]
- Qi LP, Li XT, Yang Y, et al. Multivariate Analysis of Pleural Invasion of Peripheral Non-Small Cell Lung Cancer-Based Computed Tomography Features. J Comput Assist Tomogr 2016;40:757-62. [Crossref] [PubMed]
- Zhao LL, Xie HK, Zhang LP, et al. Visceral pleural invasion in lung adenocarcinoma ≤3 cm with ground-glass opacity: a clinical, pathological and radiological study. J Thorac Dis 2016;8:1788-97. [Crossref] [PubMed]
- Ma J, Yang YL, Wang Y, et al. Relationship between computed tomography morphology and prognosis of patients with stage I non-small cell lung cancer. Onco Targets Ther 2017;10:2249-56. [Crossref] [PubMed]
- Liu QX, Deng XF, Zhou D, et al. Visceral pleural invasion impacts the prognosis of non-small cell lung cancer: A meta-analysis. Eur J Surg Oncol 2016;42:1707-13. [Crossref] [PubMed]
- Adachi H, Tsuboi M, Nishii T, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:691-7; discussion 697. [Crossref] [PubMed]
- Oyama M, Miyagi Maeshima A, Tochigi N, et al. Prognostic impact of pleural invasion in 1488 patients with surgically resected non-small cell lung carcinoma. Jpn J Clin Oncol 2013;43:540-6. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:160-5. [Crossref] [PubMed]
- Zhang H, Lu C, Lu Y, et al. The predictive and prognostic values of factors associated with visceral pleural involvement in resected lung adenocarcinomas. Onco Targets Ther 2016;9:2337-48. [Crossref] [PubMed]
- Ye Z, Zhang X, Luo Y, et al. Prognostic Values of Vimentin Expression and Its Clinicopathological Significance in Non-Small Cell Lung Cancer: A Meta-Analysis of Observational Studies with 4118 Cases. PLoS One 2016;11:e0163162. [Crossref] [PubMed]
- Xing R, Chen KB, Xuan Y, et al. RBX1 expression is an unfavorable prognostic factor in patients with non-small cell lung cancer. Surg Oncol 2016;25:147-51. [Crossref] [PubMed]
- Gupta P, Sharma PK, Mir H, et al. CCR9/CCL25 expression in non-small cell lung cancer correlates with aggressive disease and mediates key steps of metastasis. Oncotarget 2014;5:10170-9. [Crossref] [PubMed]
- Nitadori JI, Colovos C, Kadota K, et al. Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma ≤2 cm: a proposal to reclassify T1 lung adenocarcinoma. Chest 2013;144:1622-31. [Crossref] [PubMed]
- Hsu JS, Han IT, Tsai TH, et al. Pleural Tags on CT Scans to Predict Visceral Pleural Invasion of Non-Small Cell Lung Cancer That Does Not Abut the Pleura. Radiology 2016;279:590-6. [Crossref] [PubMed]
- Bartling B, Desole M, Rohrbach S, et al. Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. FASEB J 2009;23:1510-20. [Crossref] [PubMed]


