Ivor-Lewis esophagectomy for esophageal cancer after distal gastrectomy
Introduction
We have encountered a few patients who have developed esophageal cancer following distal gastrectomy. In these cases, esophageal cancer including malignant changes in the esophageal mucous membrane occurred in patients in whom distal gastrectomy was performed to treat benign gastrointestinal diseases. Esophageal cancer can also develop in patients who undergo early radical gastrectomy for gastric cancer. Normally, this condition develops in patients after five years (1). When gastrectomy is performed, the chances of developing esophageal cancer increase as postgastrectomy reflux or nutritional changes are seen in patients post-operatively (2). Moreover, reconstruction can pose a serious problem after subtotal esophagectomy in patients who have previously undergone gastric resection. Substitution of the gastric tube using the colon or jejunum is avoided as it requires complicated operative procedures. Moreover, it also leads to higher operative morbidity and mortality (3,4). Thus, there are serious problems associated with esophageal cancer surgery in patients with previous gastrectomy. In these patients, surgeons are unsure whether the gastric tube should be used to replace the esophagus as there is a risk of anastomotic leakage induced by insufficient blood flow.
Case report
A 53-year-old male underwent distal gastrectomy in 1985 due to a duodenal ulcer, which was treated with a Billroth II anastomosis. When he was referred to our hospital, he complained of progressive dysphagia for solid food for approximately four months. Alcohol abuse was the most pertinent factor in his medical history. Physical examination of the chest and abdomen did not reveal any abnormal findings. All the laboratory data, including tumor markers, were within the normal limits.
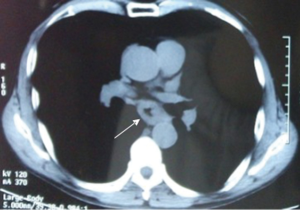
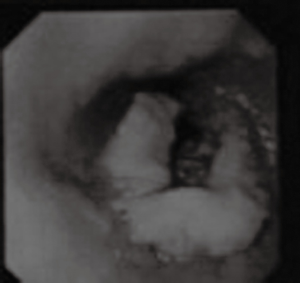
Esophagography revealed an arc filling defect approximately 7 cm in longitudinal diameter in the middle-lower esophagus (Figure 1). Moreover, there was local mucosal damage and wall stiffness. Barium esophagography also showed a capacious residual stomach with efficient emptying through a wide gastrojejunostomy. The gastric remnant was about 12 cm in length at the lesser curvature. Computed tomography (CT) of the chest showed irregular wall thickening at the middle third esophagus (Figure 2). Endoscopy confirmed a 7.0 cm bulge mass occupying more than half the circumference of the middle-third of the esophagus with the upper margin 28 cm from the dental arcade (Figure 3). The lesion did not stain with Lugol’s solution. Pathological studies of the biopsy specimens showed squamous cell carcinoma. Exclusion of cerebral, abdominal, skeletal, lymph node, and other distant metastases (M1) was accomplished using positron emission tomography-CT (PET-CT). Distant metastasis in other organs was not observed. The interval between previous distal gastrectomy and esophagectomy was 28 years.
Before surgery, we examined the entire colon using an endoscope; the bowel was prepared by mechanical cleansing. The abdomen was entered through a median incision above the umbilicus. Strict adhesions were found between the gastric remnant and adjacent organs. The right gastroepiploic artery and right gastric artery were divided at the time of previous gastrectomy, and the left gastric artery and short gastric artery were divided at their origin. Abdominal lymph node dissection was performed. The remnant stomach was freed up to the gastroesophageal junction. It appeared rosy with adequate blood supply, which may be associated with the efferent jejunal flap and its wide gastrojejunal anastomosis (Figure 4A). We decided to preserve the remnant stomach for use as a substitute organ. The jejunum was freed from the surrounding tissues. A side-to-side anastomosis was performed 10-15 cm from the afferent and efferent loops, in order to increase the lifting height of the remnant stomach and jejunal loop (Figure 4B). The abdomen was then temporarily closed. The prepared remnant stomach was pulled up with the afferent and efferent loops through the posterior mediastinal route into the right thoracic cavity.
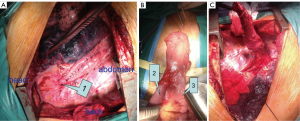
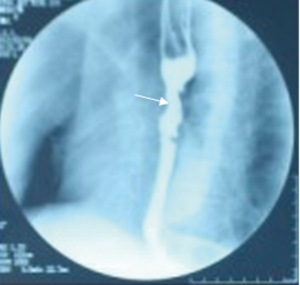
En bloc subtotal esophageal resection was performed via a posterolateral right thoracotomy through the fifth intercostal space with lymph node dissection in the mediastinum. The upper end of the esophagus was resected 5 cm above the tumor (Figure 4C), and safety of the surgical margins was confirmed by frozen sections. A gastric tube was extended far enough to reach the proximal esophagus. An esophagogastric anastomosis was performed mechanically in the apex of the chest using a circular stapler: anterior gastrotomy was performed for this purpose up to the high point of the fundus in the gastric remnant. The gastric remnant was placed in the right thoracic cavity. Microvascular anastomosis was not performed. Enteral nutrition therapy was administered using a stoma of jejunum. A nasogastric tube was positioned and remained in place for at least five days after surgery. The operative time was 310 min, and the estimated blood loss was 375 mL. The patient recovered well without major complications. On the 9th postoperative day, the integrity of the esophagogastrostomy anastomosis was confirmed by water soluble contrast radiography. Gastroesophageal reflux was not observed and the passage of liquid was smooth on esophagography (Figure 5). Oral intake was started after esophagography. He was discharged on the 12th postoperative day without complications.
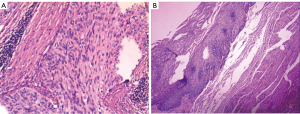
Histologically, the tumor was diagnosed as squamous cell carcinoma with tumor-free margins (Figure 6). Metastasis was not found in the abdomen or mediastinum lymph nodes. According to the 7th edition of the UICC-TNM Classification of Malignant Tumors, the pathological stage was pT3N0M0 (5). The patient was followed up for three months and did not show any evidence of recurrence. He had improved intake of food; the patient’s diet was mainly semi-solid foods. Barium esophagography confirmed good vascularity of the jejunal flap and gastric remnant.
Discussion
Traditionally, after subtotal gastrectomy for the treatment of esophageal cancer, the esophagus is replaced either with the colon or the jejunum. One of the disadvantages of colon interposition is that a long operative time is required for colon mobilization. Moreover, an additional anastomosis is required to perform colon interposition and this is quite disadvantageous. Both these disadvantages increase the surgical stress and the incidence of post-operative complications (6). Previous gastrectomy often causes strict adhesions between the mesocolon and adjacent organs; this makes the use of colon for reconstruction difficult. However, a reduction in the number of surgical maneuvers below the transverse colon and few bowel anastomoses represent real advantages (7).
There are various factors implicated in anastomotic leakage, including blood supply, physical tension, surgical procedure (hand or staple sewing) and systemic nutrition. In this study, the blood supply seemed to be sufficient. The remnant stomach lasted more than five years wherein the duodenum or jejunum vessels established a good collateral circulation. The remnant stomach blood supply may be maintained by the reconstituted microvascular supply arising from either the widely anastomosed jejunal loop or vascular adaptation of the stomach and anastomotic site (7). Reavis showed that the delay effect is associated with both vasodilation and angiogenesis, which results in increased blood flow to the gastric fundus prior to esophagogastric anastomosis in animals: delayed operations are characterized by less anastomotic collagen deposition and ischemic injury compared with immediate resection (8). Establishment of an effective collateral circulation ensures that there is adequate blood supply to accelerate healing of the anastomotic stoma. Another possible factor which can impact the anastomosis is the tensile force on the anastomosis. The length of lifted jejunum is the most practical problem during reconstruction. In the present case, the jejunum was freed from surrounding tissues. A side-to-side anastomosis was performed 10-15 cm from the afferent and efferent loops, in order to increase the lifting height of the remnant stomach and jejunal loop.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Shimada H, Okazumi S, Matsubara H, et al. Is the surgical stress associated with worse survival in patients with esophageal cancer-analysis of colon substitution for 37 patients with remnant stomach. Hepatogastroenterology 2007;54:791-5. [PubMed]
- Aiko S, Yoshizumi Y, Sugiura Y. Clinical characteristics of esophageal cancer after gastrectomy and the pertinence of chemoradiotherapy. J Jpn Surg Assoc 2002;63:813-20.
- Kolh P, Honore P, Degauque C, et al. Early stage results after oesophageal resection for malignancy-colon interposition vs. gastric pull-up. Eur J Cardiothorac Surg 2000;18:293-300. [PubMed]
- Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg 2003;138:303-8. [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Wada H, Doki Y, Nishioka K, et al. Clinical outcome of esophageal cancer patients with history of gastrectomy. J Surg Oncol 2005;89:67-74. [PubMed]
- Dionigi G, Dionigi R, Rovera F, et al. Reconstruction after esophagectomy in patients with [partial] gastric resection. Case report and review of the literature of the use of remnant stomach. Int Semin Surg Oncol 2006;3:10. [PubMed]
- Reavis KM, Chang EY, Hunter JG, et al. Utilization of the delay phenomenon improves blood flow and reduces collagen deposition in esophagogastric anastomoses. Ann Surg 2005;241:736-45; discussion 745-7. [PubMed]