Primary small cell cancer of the esophagus: understanding treatment outcomes
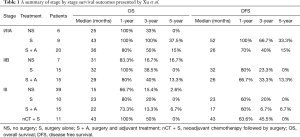
In their recent paper Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus, Xu and colleagues (1) explored the characteristics and outcomes of patients treated for primary small cell carcinoma of the esophagus (PSCCE). The authors retrospectively reviewed 152 patients with small cell cancer of the esophagus treated over 9 years at their institution, including 100 patients who received surgery (with or without chemotherapy or radiation) and 52 patients treated without resection. They concluded that treatment modality and lymph node staging status were independent prognostic factors. They performed detailed subgroup analyses of treatment strategy by stage, and a summary of these results is shown here in our Table 1 for reference. Based on their analyses, they concluded: that stage I/IIA patients should be treated with surgery; that post-resection adjuvant therapy offered no benefit in stage IIB; and that stage III patients would be best treated with neoadjuvant chemotherapy followed by surgery.
Full table
The authors should be commended for their efforts in attempting to determine the optimal treatment strategies in this rare population of patients. In-depth retrospective reviews of institutional cohorts like these can be helpful in examining treatment outcomes in uncommon conditions where randomized controlled trials are not practical. In this study, the authors provided a detailed description of how esophageal small cell cancer patients are being treated stage by stage when the diagnosis is known in advance of treatment planning. This detailed information in a large cohort is a valuable contribution to the small body of literature on this topic.
A recent meta-analysis showed that most publications on esophageal small cell cancer are case reports, and there are few larger cohorts available for review (2), so this series is certainly a useful addition. The existing evidence has suggested that small cell of the esophagus, similar to small cell cancer of the lung, is an aggressive disease with early systemic spread. Overall, patients appear to benefit from both systemic chemotherapy and regional control of the disease through surgical resection or radiation (2). Previous retrospective studies have expressed contradictory conclusions about the benefits of local versus systemic treatment (3-5). The aim of Xu and colleagues in this work, to compare outcomes by stage to develop evidence-based treatment recommendations, is laudable, and their subgroup analyses are performed and presented in a logical manner.
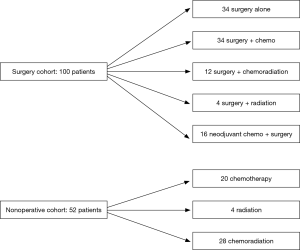
Although this study provided detailed survival data for specific treatment strategies by stage of disease, one limitation of this report is the challenge of determining precisely to whom these results may generalize. The inclusion criteria of “proven limited stage” and exclusion criteria of “uncontrolled comorbid conditions” and “incomplete medical records” were vague, and the number of patients excluded for these reasons was not given. For the analyzed patients, characteristics were presented for the entire cohort, but not broken down by treatment modality. Significant heterogeneity in treatment regimens was also present in both the surgical and nonoperative cohorts, and is shown in our Figure 1. In addition to the varied use of chemotherapy, radiation, or both, the authors also acknowledge that the specific agents used, dosages given, and cycles administered were not consistent. These represent unknown variables that certainly could have affected the reported outcomes. Inclusion of this information would have allowed for thoughtful comparison of the types of patients receiving surgery versus nonoperative management.
There was a significant risk of several different types of bias in their survival analyses stratified by treatment, not only because of stage differences, as the authors have acknowledged, but also because of differences in comorbidities, functional status, and fitness for surgery that exist between the treatment groups. As one examines results summarized here in our Table 1, it seems clear that there was selection bias introduced by non-random allocation of treatment, particularly with regards to administration of adjuvant therapy. Across all three stage groups, patients given adjuvant therapy after surgery had worse overall survival, which might be explained by toxic effects of chemotherapy or radiation, but also worse disease-free survival than patients undergoing surgery alone. The latter finding cannot be explained by positive or negative effects of chemoradiation. This indicates that adjuvant therapy was not administered at random after surgery: almost certainly, some aspects of each patient’s cancer biology factored into this decision for more therapy. These factors may have included high-risk features on histologic exam or positive margins after resection and these unreported covariates may account for the observed poorer prognosis of this subgroup. In addition to selection bias, the reported survival analyses are subject to “immortal time bias” (6). Patients receiving neoadjuvant chemotherapy followed by surgery may appear to have improved survival in part because patients who suffered cancer progression or became too sick for surgery during their induction treatment are excluded from this cohort. These poor performing recipients of induction therapy would be considered instead in the ‘no surgery’ group. Moreover, patients treated surgically were staged pathologically whereas patients treated nonoperatively were staged clinically. As in small cell cancer of the lung, there is early subclinical nodal spread, and it’s possible the nonoperative small cell of the esophagus patients were clinically understaged, further explaining the worse prognosis seen in that group. Interpretation of the survival analyses in this paper must be done with an awareness of these biases.
Finally, despite this being one of the largest cohorts of esophageal small cell cancer described, there is still a relatively small number of patients, even before dividing these individuals up into subgroups for analysis. As a result of the small sample size, the study has low power and a high risk of a type II error, or failing to detect a difference in outcome where there is one. This issue of low statistical power is relevant to the conclusion that adjuvant therapy does not improve overall survival, and is a limitation of other papers mentioned in their discussion as well. The authors appropriately acknowledged some of these concerns in their discussion, and the conclusions regarding optimal treatment should be tempered considerably in light of these limitations.
Though this article doesn’t solve the controversy regarding optimal treatment for PSCCE, it does provide additional useful observational data on outcomes in a cohort of patients with this rare disease. The study supports the idea that surgery can be used for locoregional control, and it provides detailed prognostic data that can be helpful in counseling patients facing treatment decisions. While further studies are needed to clarify which modalities will lead to the best outcomes, Xu and colleagues are congratulated for contributing their unique experience to a small body of published work that providers can use to make individualized treatment decisions for patients with primary small cell carcinoma of the esophagus.
Acknowledgements
Funding: This work was supported by National Institutes of Health (NIH) Grant Number 2T32HL7776-21, a Barnes Jewish Hospital Foundation Grant, and the Division of Cardiothoracic Surgery at Washington University in St. Louis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Xu L, Li Y, Liu X, et al. Treatment strategies and prognostic factors of limited-stage primary small cell carcinoma of the esophagus. J Thorac Oncol 2017;12:1834-44. [Crossref] [PubMed]
- Raja S, Rice TW, Rajeswaran J, et al. Esophageal small-cell cancer: study of a rare disease. Dis Esophagus 2013;26:690-5. [PubMed]
- Situ D, Lin Y, Long H, et al. Surgical treatment for limited-stage primary small cell cancer of the esophagus. Ann Thorac Surg 2013;95:1057-62. [Crossref] [PubMed]
- Chen WW, Wang F, Chen S, et al. Detailed analysis of prognostic factors in primary esophageal small cell carcinoma. Ann Thorac Surg 2014;97:1975-81. [Crossref] [PubMed]
- Hosseini S, Salek R, Nasrolahi H, et al. Small cell carcinoma of the esophagus: clinicopathological features and outcome of 22 cases. Iran Red Crescent Med J 2015;17:e20353. [Crossref] [PubMed]
- Ho AM, Dion PW, Ng CS, et al. Understanding immortal time bias in observational cohort studies. Anaesthesia 2013;68:126-30. [Crossref] [PubMed]