Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery
Introduction
Theoretical advantages of limited resection for early stage lung cancer include preservation of pulmonary function, increased potential for a second resection with a subsequent primary tumor, and potentially similar oncologic results (1,2). While lobectomy has been established as the standard procedure for early stage lung cancer, limited resections were only considered in patients unable to tolerate lobectomy (2). However, with the use of computed tomographic (CT) screening, increasingly more small pulmonary nodules are detected, especially ground glass opacities (GGOs) associated with favorable histology (3). This has resulted in a reviving interest in limited resections recently. Several retrospective reports have demonstrated that VATS segmentectomy for stage IA lung cancer may have a prognosis and local recurrence rate comparable to VATS lobectomy (4,5). However, pulmonary functional benefit of this less invasiveness intended procedure is still dubious, as most available data were based on open thoracotomy (6-8).
The purpose of this study was to compare postoperative changes of pulmonary function in patients undergoing VATS lobectomy or limited resections, and to evaluate the degree of pulmonary function change associated with extent of resection.
Methods
Patient
Patients who underwent VATS for clinically suspected stage IA non-small cell lung cancer (NSCLC) at Shanghai Chest Hospital between November 2011 and March 2014 were prospectively included in an observational study. The study was approved by the Institutional Review Board of the hospital (Number/ID of the Ethic Approval is ks11014). Informed consent was acquired from patients for using characterized information for clinical study. To evaluate the effect of thoracoscopic incision on pulmonary function, 11 VATS mediastinal procedures without lung resection were taken as a control group. All patients included in this study were functionally fit for standard lobectomy, and those with severe comorbidity or significantly compromised pulmonary function before surgery who received limited resections as a compromised procedure were not included. A wedge resection was performed based on the following criteria: (I) a suspicious lesion (with CT findings) <2.0 cm in diameter located in peripheral; (II) frozen-section analysis of the lesion is atypical adenomatous hyperplasia (AAH) or adenocarcinoma in situ (AIS). Indications for segmentectomy were peripheral lesions located in close proximity to segmentary bronchial structures. Thoracoscopic lobectomy or multiple segmentectomy was done for a lesion located on the edge of the diseased segment. During thoracoscopic wedge resection, conversion to segmentectomy or lobectomy was indicated when the frozen section of lesion shown to be invasive adenocarcinoma or squamous carcinoma. A systematic nodal dissection or sampling is performed in all segmentectomies and lobectomies. All procedures were performed via three-port VATS under general anesthesia with single-lung ventilation. During segmentectomy, the segmental pulmonary veins, arteries, and bronchi were dissected and stapled separately. An endoscopic stapler was used to divide the intersegmental plane according to the inflation-deflation line. Full description of this technique has been previously published (4). No patient received preoperative chemotherapy or radiation. Patients having major postoperative complications, or those receiving adjuvant radiation and/or chemotherapy after surgery were excluded from the study for fear that postoperative treatment might have some impact on pulmonary function recovery. Patients with middle lobectomy were not included, as there was no middle lobe segmentectomy in this group.
Pulmonary function tests
Spirometry tests were obtained prospectively before and 6 months after surgery (Sensormedics Vmax 6229, USA) according to American Thoracic Society standards (9). Pulmonary function studies included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and diffusion capacity to carbon monoxide (DLCO). The loss in pulmonary function variables at 6 months after surgery was measured according to the following formula (take FVC as an example). FVC loss = (preoperative FVC − postoperative FVC)/preoperative FVC ×100%. To rule out the potential effect of number of segments in each lobectomy or segmentectomy, average pulmonary function loss per segment resected was also calculated according as following (again take FVC as an example) Average FVC loss per segment resected = FVC loss/number of segments resected.
Statistical analyses
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). All data are expressed as means ± standard deviation. Differences between groups with categorical variables were assessed by the χ2 test or the Fisher exact test. Continuous data were analyzed with the Student’s t-test. The analysis of variance was used for the intergroup comparison. Statistical significance was accepted as a P value of less than 0.05 throughout the study.
Results
Altogether 135 patients were enrolled in this study, 75 undergone VATS lobectomy, 34 VATS segmentectomy, and 15 VATS wedge resection. Eleven VATS mediastinal procedures without lung resection were taken as a control group. Table 1 shows the location of the lesions. Patient characteristics are shown in Table 2. There were no significant differences in demographic characteristics or preoperative pulmonary function among these groups. Mean number of segments resected in the lobectomy group was 3.6±0.7, which was significantly more than the segmentectomy group (1.6±0.5, P<0.01).

Full table

Full table
Postoperative changes of pulmonary function are shown in Table 3. Although no difference was detected when comparing postoperative spirometry among the four groups, significant differences were noticed when loss of pulmonary function after surgery was compared. FVC loss after lobectomy was significantly greater than after segmentectomy (P=0.048), and much significantly greater than after wedge resection (P<0.001, Figure 1). FEV1 loss after lobectomy was similar to segmentectomy (P=0.273), both significantly greater than after wedge resection (P<0.01, Figure 2). DLCO loss was similar among these three groups (P=0.316, Figure 3). There was no significant difference in any spirometry change between wedge resection and mediastinal procedures (FVC: P=0.856; FEV1: P=0.671; DLCO: P=0.057).

Full table
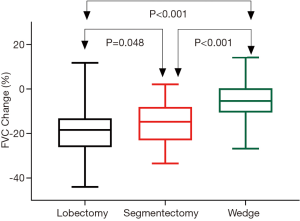
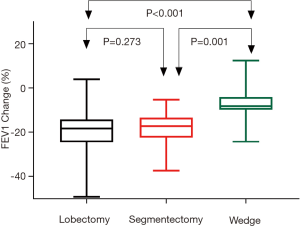
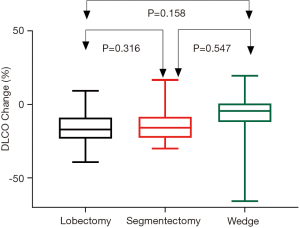
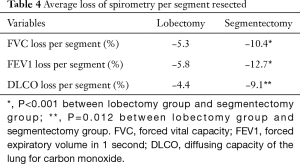
Table 4 shows the average loss of pulmonary function per segment resected in lobectomy and segmentectomy groups. Significantly greater loss in FVC (P<0.001) and FEV1 (P<0.001), as well as in DLCO (P=0.001) was detected after segmentectomy than after lobectomy. On average, FVC, FEV1 and DLCO loss would be around 5% per segment resected for VATS lobectomy and 10% per segment resected for VATS segmentectomy.
Full table
Discussion
Limited resection is an attractive alternative to lobectomy for early stage NSCLC recently (10,11). However, oncologic and functional benefit of this less invasive intended procedure is still ill defined (4,5,12). The only published prospective randomized study that compared lobectomy versus limited resection of stage IA NSCLC was reported in 1995 by the Lung Cancer Study Group (LCSG). The study found no statistical evidence for preservation of pulmonary function (except a modest benefit in FEV1) compared with lobectomy (12). Takizawa and colleagues reached similar result in 1999 (7). Harada (13) and Saito (8), respectively, also found that loss of FVC and FEV1 were less after segmentectomy than after lobectomy. But all these studies compared open segmentectomy with after open lobectomy (6,13,14). And no study has ever compared pulmonary function changes after VATS lobectomy and VATS sublobar resections, especially in good risk patients who could tolerate a lobectomy.
Our study showed that pulmonary function at 6 months after VATS segmentectomy was only slightly better than that after VATS lobectomy, although significantly less lung parenchyma was resected in the segmentectomy group than in the lobectomy group (3.68±0.76 segments vs. 1.59±0.5 segments, P<0.01). And VATS wedge resection had similar impact on FVC and FEV1 comparing to mediastinal procedures without pulmonary resection, representing functional loss caused by mere VATS incisions.
Comparing to segmentectomy, FVC loss (−19.19% vs. −15.03%,P=0.048) but not FEV1 loss (−21.02% vs. −18.39%,P=0.273), was statistically significant after lobectomy. This was different with previous studies with open thoracotomy (6-9,12,13). The possible reasons could be as follows: (I) change in FVC is mainly determined by amount of lung tissue resected, which is significantly different between the lobectomy and segmentectomy groups (3.68 vs. 1.59, P<0.01). But change in FEV1 may be related more to ventilation mechanisms including existing airway obstruction, compensatory expansion of the residual lung, and chest wall activity. Given that all patients included in this study were good risk candidates for surgery, the latter two might play a more important role in FEV1 change; (II) the benefit of VATS approach is likely to offset some disadvantages caused by resection of pulmonary parenchyma (minimization of chest wall destruction and deformity, less incision pain), as compared to open thoracotomy; (III) more than half of the patients in the segmentectomy group (19/34) had more than one segment resected. And on average 1.59 segments were taken out in this group. Combined segmentectomy would certainly cost more functional loss than single segmentectomy.
We further compared the average loss of pulmonary function per segment resected between lobectomy and segmentectomy groups. This was found to be significantly greater after segmentectomy than after lobectomy. Average loss of spirometry indexes was approximately 10% after VATS segmentectomy, significantly greater than after VATS lobectomy, which was only around 5% per segment resected. This may be explained by relatively less satisfactory re-expansion of the residual lung after segmentectomy, as the intersegmental plane was divided by staplers in our patients. While stapling of the intersegmental plane during segmentectomy may have helped reducing air-leak problems, it may also restrict re-expansion of the residual segments in the remaining lobe (15). This result should be carefully considered when predicting postoperative pulmonary function and when considering segmentectomy for high risk patients. It seems that postoperative functional benefit could be expected only if at least more than half of the lung parenchyma in the corresponding lobe is preserved. And it is less likely for resection of more than half of the segments in a lobe, like in the case of lingual-sparing left upper lobectomy, to have significant functional gain comparing to a standard lobectomy.
Contrary to ventilation functions, changes in diffusion function after pulmonary resection was seldom explored in previous studies (6). The DLCO reflects the capillary surface area available for gas diffusion, indicating the ability to oxygenation of the lung. Our study showed that from high to low, the order of DLCO loss was lobectomy, segmentectomy, and wedge resection, although no statistical significances were found in the differences. As opposed to decrease of DLCO after lung resection, little has changed in DLCO after VATS mediastinal procedures. DLCO loss after VATS wedge resection was higher than that after VATS mediastinal procedures with a marginal significance (P=0.057). This suggested that DLCO loss might be associated with the mechanical injury of residue lung during surgery. With the increase of case sample, the result might become statistically significant.
Comparing to VATS lobectomy or segmentectomy, VATS wedge resection could best preserve postoperative pulmonary function. Our results showed that mere incisions of VATS would cause nearly 5% FVC and FEV1 loss. When compared with VATS mediastinal procedures without lung resection, VATS wedge resection had similar spirometry changes at 6 months after surgery. Apart from preserving more lung tissue, a less invasive nature of the procedure was clearly demonstrated. Meanwhile, although this study was carried out in good risk patients, the favorable functional results after VATS wedge resection were also helpful when deciding the extent of resection for patients with poor pulmonary function.
The major limitation of this study is the relatively small sample size of limited resection group, making it impossible to study single segmentectomies alone. Currently we are accruing more patients so as to better study the effect of extent of resection on postoperative pulmonary function. Also, we didn’t evaluate the impact of pulmonary resection on the quality of life (QoL) of patients. Considering the QoL is as important as pulmonary function changes when deciding the extent of resection for patients with lung cancer, further studies are needed to address this issue. Moreover, our study was carried out solely in good risk patients who could have tolerated lobectomy in the first place. The conclusions may not be extrapolated to high-risk patients with compromised cardiopulmonary functions. However, to our knowledge, this is the first prospective study focusing on pulmonary function changes after VATS lobectomy and limited resections, including both segmentectomy and wedge resection. Also, we studied not only spirometry change in general, but pulmonary function loss per segment resected so as to better evaluate the extent of resection on functional changes.
In conclusion, VATS wedge resection could best preserve pulmonary function, with similar spirometry change with VATS mediastinal procedures without lung resection. Compared with VATS lobectomy, VATS segmentectomy might help minimize loss of FVC but not FEV1 or DLCO. Pulmonary function loss per segment resected is doubled after VATS segmentectomy than after lobectomy. These results should be taken into account when deciding the extent of resection for patients with early stage lung cancer.
Acknowledgements
Funding: This work was supported by the Science and Technology Commission of Shanghai Municipality (No. 14411950800).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the hospital (Number/ID of the Ethic Approval is ks11014). Informed consent was acquired from patients for using characterized information for clinical study.
References
- Oizumi H, Kanauchi N, Kato H, et al. Total thoracoscopic pulmonary segmentectomy. Eur J Cardiothorac Surg 2009;36:374-7; discussion 377. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8.
- Henschke CI, Yankelevitz DF, Altorki NK. The role of CT screening for lung cancer. Thorac Surg Clin 2007;17:137-42. [Crossref] [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Takizawa T, Haga M, Yagi N, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536-41. [Crossref] [PubMed]
- Saito H, Nakagawa T, Ito M, et al. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J Surg 2014;38:2025-31. [Crossref] [PubMed]
- Standardization of spirometry--1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987;136:1285-98. [Crossref] [PubMed]
- Mineo TC, Tacconi F, Ambrogi V, et al. Nonintubated VATS segmentectomy: when and for whom? Ann Thorac Surg 2014;98:388. [Crossref] [PubMed]
- Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol 2014;9:383-9. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Asakura K, Izumi Y, Kohno M, et al. Effect of cutting technique at the intersegmental plane during segmentectomy on expansion of the preserved segment: comparison between staplers and scissors in ex vivo pig lung. Eur J Cardiothorac Surg 2011;40:e34-8. [Crossref] [PubMed]


