Tumor-infiltrating CD45RO+ memory cells correlate with favorable prognosis in patients with lung adenocarcinoma
Introduction
Lung cancer, the leading cause of cancer-related death worldwide, has a high incidence and mortality rate (1). The main classification of lung cancer includes small cell lung carcinoma (SCLC) and non-small cell lung cancer (NSCLC) (2). NSCLC, which accounts for 85% of all lung cancers, primarily comprises adenocarcinoma, squamous cell carcinoma, large cell carcinoma, sarcomatoid carcinoma, and adenosquamous cell carcinoma, among which lung adenocarcinoma is one of the most aggressive histological types in lung cancer and is the main subtype of NSCLC (3). Despite the improvement in lung adenocarcinoma treatments, surgical resection is still the primary option for patients with lung adenocarcinoma; however, most clinically diagnosed cases are inoperable because of metastasis or present poor prognosis after surgery (4). Therefore, additional novel and convincing biological markers, which are helpful to indicate the diagnosis and prognosis for lung cancer, are urgently needed.
Although the pathogenesis of lung adenocarcinoma is complicated and obscure, genetic features, environmental factors and dysregulated living habits greatly contribute to the development and progression of this disease (5,6). Among these factors, the development of lung adenocarcinoma is controlled by a biological system that depends on genetic factors as well as the interplay between tumor cells, stromal cells, and host immune cells (7). Immune system invasion and/or escape is essential in the etiology of lung adenocarcinoma, and the presence of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment is an indication of the host immune response to tumor antigens (8). TILs comprise a variety of immune cells, including CD4+ T lymphocytes, CD8+ lymphocytes, FOXP3+ regulatory T cells, natural killer (NK) cells, dendritic cells and macrophages (9). TILs are attracting attention for their use in monitoring the immune response in the tumor microenvironment and predicting the prognosis and treatment response in several cancers, such as colorectal and breast cancers (8,9).
Therefore, in the present study, we proposed that the infiltration of lymphocytes in lung adenocarcinoma and expression of chemokine receptors might be correlated with tumor differentiation, TNM stage, clinical stage, disease-free survival (DFS) or overall survival (OS). We aimed to evaluate the infiltration of CD45RO+, CD8+, CCR7+ and FOXP3+ cells in paraffin-embedded lung adenocarcinoma tissues and examine their correlation with clinicopathological parameters as well as prognosis.
Methods
Patients
Ninety patients with lung adenocarcinoma who underwent the surgery at Shanghai Chest Hospital (Shanghai, China) between Jan. 2007 and Jun. 2011 were consecutively recruited in the present cohort study. All patients were diagnosed with lung adenocarcinoma based on clinical manifestations, radiological and histological findings (Table 1), and no patients had received new adjuvant therapies, such as radiotherapy or chemotherapy, prior to surgery. Lung adenocarcinoma and paired adjacent lung tissues were obtained from all participants, and clinical as well as pathological data were collected. In the present study, the number of patients with lobectomy, local resection and pneumonectomy were 75 (83%), 10 (11%) and 5 (6%), respectively. In addition, among the 78 patients with stage IB–IV, 63 (80.8%) patients received adjuvant chemotherapy and 15 (19.2%) patients did not. The present study was approved by the Institutional Review Board for Clinical Research of the Shanghai Chest Hospital. Written informed consent was obtained from all subjects prior to the study. The number/ID of the Ethic Approval was ILU02.
Full table
Follow-ups
All patients were followed up by clinic visits or telephone calls, once a month in the first half year, once every 3 months from 0.5 to 2 years, and then once every 6 months from 2 years to the last date of follow up (2016/08/20). OS and DFS were calculated from the surgery to the time of death from any cause, and the time of documented local or distant recurrence of the initial cancer, respectively. Five patients were lost to follow-up during the entire study, with follow-up durations of 8, 40, 48, 52 and 64 months, respectively. The median follow-up duration was 46.0 months (ranging from 1 to 121 months).
Immunohistochemistry
Prior to immunohistochemistry analysis, all the sections were stained with hematoxylin and eosin and reviewed to confirm the histopathological diagnosis. As previously described (10), paraffin-embedded lung adenocarcinoma and paired adjacent lung tissue specimens were cut into 4-µm sections and mounted on poly-L-lysine-coated glass slides. Following deparaffinization and rehydration, antigen retrieval was performed by autoclaving the sections in 10 mmol/L Tris-EDTA buffer (pH 9.0) at 121 °C, subsequently endogenous peroxidase was blocked using 3% H2O2, and the sections were immersed in 4% bovine serum albumin. The sections were then incubated with a 1:100 dilution of rabbit or mouse monoclonal antibody against human CD45RO, CD8, CCR7 and FOXP3 (All from Abcam, USA) at 4 °C overnight according to the manufacturer’s instructions. For negative controls, sections were treated with phosphate-buffered saline (PBS) instead of primary antibodies. After washing with PBS, the sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at 37 °C for 1 hour. Subsequently, the signal was detected using DAB and hematoxylin was used for counterstaining. One hundred cells from five selected representative high-power fields (HPF, ×400) of each section were independently counted by pathologists without prior knowledge of the clinicopathological data for the determination of the immunostaining intensity. The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong), while the grading scale for labeling frequency ranged from 0 (0%) to 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%) on the basis of the percentage of positively stained cells. After multiplying the staining intensity score by the labeling frequency score, the sections were divided into three groups: TILs low (final score ≤3), TILs intermediate (3< final score ≤6) and TILs high (final score >6).
Statistical analysis
The data were presented as the means ± standard deviation (SD), count (percentage). Chi-square test was used to compare CD45RO+, CD8+, CCR7+ and FOXP3+ infiltrating lymphocytes among the different groups. DFS and OS were analyzed by the Kaplan-Meier method. In addition, univariate Cox’s proportional hazards regression model was performed to determine predictors of DFS as well as OS in lung adenocarcinoma patients, while all factors with a P value less than 0.1 were subsequently included in the multivariate Cox’s proportional hazards regression model to investigate the independent predictive factors. Statistical analysis was performed using the SSPS 21.0 program and a P value <0.05 was considered significant.
Results
Patient characteristics
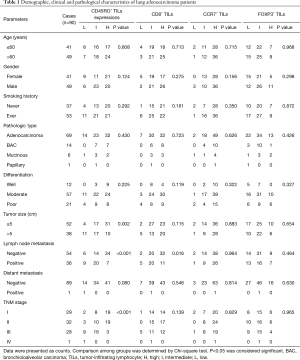
Among the 90 patients with a median age of 61.5 [55–71] years, 49 (54%) patients were males. Twelve cases (13%) were well differentiated, while 57 (63%) and 21 (24%) cases were moderately and poorly differentiated, respectively. A total of 29 (32%), 32 (36%), 28 (31%), and 1 (1%) cases were TNM stages I, II, III, and IV, respectively. The other baseline characteristics of the lung adenocarcinoma patients are summarized in Table 1.
CD45RO+, CD8+, CCR7+ and FOXP3+ infiltrating lymphocytes in lung adenocarcinoma and adjacent lung tissues
To explore the role of immune cells in the tumor microenvironment, we examined the expression of different immune cell proteins by immunohistochemistry. As shown in Figure 1A, CD45RO was mainly expressed in immune cells infiltrated in adenocarcinoma tissues and predominantly present in the cell membrane. CD45RO+ infiltrating lymphocytes were markedly increased in lung adenocarcinoma than those in adjacent lung tissues (P<0.001) (Table 2). Consistent with the increased CD45RO+ infiltrating lymphocytes, CD8 and CCR7 were also expressed on the membranes of immune cells and presented a high level of expression in lung adenocarcinoma compared with that in adjacent lung tissues (both P<0.001) (Figure 1B,C, Table 2). However, FOXP3 was primarily expressed in the nucleus of immune cells and presented a decreasing tendency in lung adenocarcinoma (P<0.001) (Figure 1D, Table 2).


Full table
Correlation of CD45RO+, CD8+, CCR7+ and FOXP3+ TILs with clinicopathological features
CD45RO+ TILs were negatively associated with tumor size (P=0.002), lymph node metastasis (P<0.001) and TNM stage (P<0.001), as presented in Table 1. CD8+ TILs were also negatively correlated with lymph node metastasis (P=0.016) (Table 1). While no other correlations of CD45RO+ and CD8+ TILs with clinicopathological features were observed in the present study. CCR7+ and FOXP3+ TILs showed no association with the clinical and pathological parameters (Table 1).
Association of CD45RO+, CD8+, CCR7+ and FOXP3+ TILs with DFS and OS
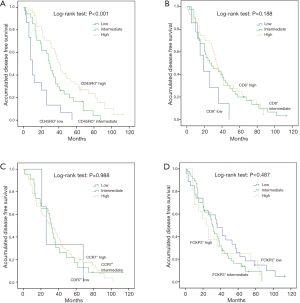
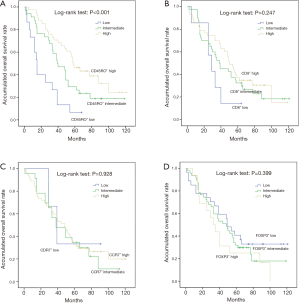
Kaplan-Meier curve analysis was performed to evaluate the effects of CD45RO+, CD8+, CCR7+ and FOXP3+ TILs on DFS and OS. As shown in Figure 2, CD45RO+ TILs were positively associated with longer DFS (P<0.001), while no association of CD8+ (P=0.188), CCR7+ (P=0.988) and FOXP3+ (P=0.487) TILs with DFS was observed. As shown in Figure 3, CD45RO+ TILs were also positively associated with better OS (P<0.001), and CD8+ (P=0.247), CCR7+ (P=0.928) and FOXP3+ (P=0.399) TILs were not correlated with OS.
Analysis of baseline factors affecting DFS
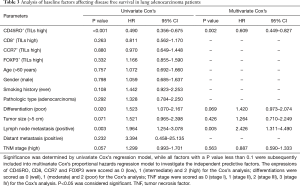
In the present study, a total of 82 (91%) patients had local (n=71) or distant (n=11) recurrence of the initial cancer, whereas 8 (9%) patients were free of disease at the end of the follow-up. To investigate the correlation of the baseline factors with DFS, a univariate Cox’s proportional hazards regression model was generated, and CD45RO+ TILs (high) (P<0.001) were determined to be predictors for longer DFS, while poor differentiation (P=0.020) and lymph node metastasis (P=0.003) were associated with shorter DFS (Table 3). All factors with P<0.1 were further analyzed by multivariate Cox’s proportional hazards regression model, and CD45RO+ TILs (high) were revealed as an independent factor for prolonged DFS (P=0.002), and on the contrary, lymph node metastasis could independently predict worse DFS (P=0.005) (Table 3).
Full table
Analysis of baseline factors affecting OS
In the present study, a total of 67 (74.4%) patients died for the high mortality of lung adenocarcinoma, whereas 23 (25.6%) patients were alive at the end of the follow-up. Univariate Cox’s proportional hazards regression model showed that CD45RO+ TILs (high) was a predictive factor for longer OS (P<0.001), whereas poor differentiation (P=0.016), tumor size (P=0.024), lymph node metastasis (P=0.001) and TNM stage (P=0.022) were correlated with short OS (Table 4). Subsequent multivariate Cox’s proportional hazards regression model showed that CD45RO+ TILs (high) independently predicted better OS (P<0.001), while poor differentiation (P=0.002), positive lymph node metastasis (P=0.005) and positive distant metastasis (P=0.024) were independent factors for worse OS (Table 4).
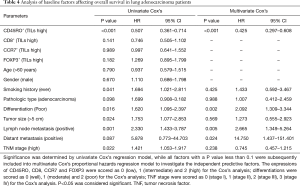
Full table
Discussion
In the present study, (I) CD45RO+, CD8+ and CCR7+ infiltrating lymphocytes were markedly increased in lung adenocarcinoma than in adjacent lung tissues, while FOXP3+ infiltrating lymphocytes were reduced; (II) CD45RO+ TILs were negatively correlated with tumor size, lymph node metastasis and TNM stage, and CD8+ TILs were only negatively associated with lymph node metastasis; (III) CD45RO+ TILs were associated with prolonged DFS and OS, as independent predictive factors for better DFS as well as OS.
The microenvironment, comprising endothelial cells and fibroblasts, structural components and infiltrating immune cells, plays a critical role in the development and progression of various cancers (11-14). Among these factors, accumulating evidence has revealed that immune cells cross-interact with tumors, leading to immune responses (15). Additionally, the immune context, defined by the type, density, location as well as organization of immune cells, is a crucial determinant of the clinicopathological features of tumors and patient prognosis (11,15). Accumulating evidence has proven that in general, tumor stroma immune cells mainly consist of TIL and tumor associated macrophages (TAM), but almost no NK cells, and a few dendritic cells. As to TIL cells, T-cells occupy nearly 80%, and approximately 30% T-cells are TIA-1 positive CD8 activated cytotoxic lymphocytes (16). TILs, as a group of heterogeneous immune cells, infiltrate tumors and reflect the immune response status between host immune cells and tumors. Previous studies have shown that the strong infiltration of TILs correlates with tumor development, individual properties, clinical response and long-term survival in several cancers, including lung cancer (17,18). Thus, in the present study, we examined the CD45RO+, CD8+, CCR7+ and FOXP3+ infiltrating lymphocytes by immunohistochemistry in lung adenocarcinoma tissues and paired adjacent lung tissues to explore their association with the occurrence, clinicopathological properties and prognosis of lung adenocarcinoma.
Some tumors, including lung adenocarcinoma, acquire the ability to damage and exploit inflammatory responses to promote the proliferation, survival, and invasiveness of tumor cells (19,20). Thus, the presence of leukocytes within tumor microenvironment may be a consequence of an inflammatory response that favors tumor development (21). CD45, also known as leukocyte common antigen, is encoded by the PRPRC gene and serves as a pan-inflammatory marker widely used in routine diagnostic pathology laboratories (22). According to the differences in the expressional features on T cells, CD45+ T cells were divided into CD45RA+ naive T cells and CD45RO+ memory T cells, and the latter could kill tumor cells by the immediate activation of immune responses (23,24). A previous study revealed that CD45RO+ T cells inhibit the proliferation and migration of cervical cancer cells by regulating interleukin (IL)-2, interferon (IFN)-γ and tumor necrosis factor (TNF)-α (25). Additionally, CD45RO+ T cells could decrease the production of high-mobility group box 1 (HMGB1), and subsequently suppress the proliferation and migration of colorectal tumor cells (26). These studies indicate the anti-tumor effect of CD45RO+ T cells. In addition, CD45RO+ TILs are negatively correlated with tumor size, lymph nodes metastasis in breast cancer patients (27), associated with lesser advanced T stage and TNM stage in rectal cancer (28), and correlated with well differentiation and lower TNM stage in renal cell carcinoma (29). Consistent with previous studies, we found that CD45RO expression was increased in lung adenocarcinoma tissues compared to paired adjacent lung tissues, and CD45RO+ TILs were negatively correlated with tumor size, lymph node metastasis and TNM stage in lung adenocarcinoma patients. These findings might result from the effect of CD45RO+ TILs on inhibiting the proliferation and migration of tumor cells through multiple mechanisms, such as excreting various cytokines, activating shrewd immune responses, etc.
Apart from the correlation with clinicopathological properties, CD45RO+ TILs (high) are associated with favorable outcomes in several cancers. In breast cancer patients, CD45RO+ TILs (high) correlates with prolonged recurrence-free survival (27), and in stage II colorectal cancer patients, CD45RO+ TILs (high) could predict better clinical response and cancer-specific survival (9). Moreover, CD45RO+ TILs are positively associated with DFS and OS in patients with renal cell carcinoma, gastric cancer and ovarian carcinoma (24,29,30). As for NSCLC patients, a previous study indicated that CD45RO+ TILs are an independent positive prognostic factor for disease-specific survival (DSS) (31). Another interesting study revealed that CD45RO+ T-cells could independently predict better DSS in NSCLC patients with subtype of squamous cell carcinoma. Consistent with previous studies of other cancers, CD45RO+ TILs (high) were associated with prolonged DFS and OS in lung adenocarcinoma patients, serving as an independent predictive factor for better DFS as well as OS. These results might be explained by (I) the direct killing effect of CD45RO+ TILs on tumor cells by repressing the proliferation and migration through activating immune responses (23-26); and (II) CD45RO+ TILs increase the efficacy of chemotherapy and/or radiotherapy, thus improving the prognosis of cancer patients (28,31).
In the present study, we also investigated the roles of CD8+, CCR7+ and FOXP3+ TILs in the development and prognosis of lung adenocarcinoma and showed that CD8 and CCR7 expression was elevated in lung adenocarcinoma tissues compared with paired adjacent lung tissues, while FOXP expression was decreased. However, only CD8+ TILs were negatively correlated with lymph node metastasis, while no correlations of these three-marker TILs with other clinicopathological features and prognosis were detected in the present study. These results might be due to relatively small samples, which resulted a lack of sufficient patients with specific TILs (high) or TILs (low), thus the statistics results showed no significance.
The relapse rate was relatively high, and the possible reasons were as follows: Firstly, the follow-up duration was relatively long in this study with the longest follow-up of 121 months (nearly 10 years). Secondly, more than half patients were with age >60 years, and the median age of these 90 patients was 61.5 [55–71] years old, which might contribute to the relatively high relapse rate. There were some limitations in the present study. Among these, the major limitation was that this was a retrospective study, we did not identify the major findings in an independent cohort of surgically resected tumors; thus, additional studies are needed. Moreover, the treatments after surgery were not analyzed, which might lead to bias during the study. However, the therapeutic options did not differ much in lung adenocarcinoma patients after surgery. In addition, the sample size was relatively small; thus, some differences or correlations might not have been observed. Few patients with CD8+ TILs (low) and CCR7+ TILs (low) were recruited, leading to a lack of efficiency in the statistical analysis for survival. Furthermore, only one distant metastasis patient was included in the present study; thus, the influence of specific TILs on prognosis in lung adenocarcinoma patients with distant metastasis was not analyzed. Finally, we performed immunohistochemistry to detect expressions of CD45RO, CD8, CCR7 and FOXP3, and during the processes of immunohistochemistry, we only marked CD45RO, CD8, CCR7 and FOXP3, without marked total T-cells, which caused that we could not know the detailed ratio of CD45RO+, CD8+, CCR7+ and FOXP3+ positive cells vs. total lymphocytes or total T-cells in this study. Thus, further study is great needed.
In conclusion, the present study demonstrates that CD45RO+ TILs are negatively correlated with tumor size, lymph node metastasis and TNM stage and that CD45RO+ TILs (high) can be regarded as a novel and promising biomarker for prolonged DFS and OS in lung adenocarcinoma patients.
Acknowledgements
Funding: The present study was financially supported through grants from the Science and Technology Commission guidance of Shanghai (No. 124119a6300), the International Science and Technology Cooperation of Shanghai (No. 14430723300), the National Natural Science Foundation of China (No. 81472642) and The Peak Plateau Project of Shanghai Jiao Tong University School of Medicine, as well as three pathologists in Shanghai Chest Hospital (Shanghai, China), including Yuchen Hang, Lei Zhu and Jinchen Shao—Research Doctor (Hua Zhong: 20161434).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Institutional Review Board for Clinical Research of the Shanghai Chest Hospital (No. ILU02). Written informed consent was obtained from all subjects prior to initiating the present study.
References
- Field JK, Oudkerk M, Pedersen JH, et al. Prospects for population screening and diagnosis of lung cancer. Lancet 2013;382:732-41. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727-40. [Crossref] [PubMed]
- Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev 2016;35:75-91. [Crossref] [PubMed]
- Swanton C, Govindan R. Clinical Implications of Genomic Discoveries in Lung Cancer. N Engl J Med 2016;374:1864-73. [Crossref] [PubMed]
- Yoon JY, Lee JD, Joo SW, et al. Indoor radon exposure and lung cancer: a review of ecological studies. Ann Occup Environ Med 2016;28:15. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Asano Y, Kashiwagi S, Goto W, et al. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 2016;103:845-54. [Crossref] [PubMed]
- Chew A, Salama P, Robbshaw A, et al. SPARC, FOXP3, CD8 and CD45 correlation with disease recurrence and long-term disease-free survival in colorectal cancer. PLoS One 2011;6:e22047. [Crossref] [PubMed]
- Husni RE, Shiba-Ishii A, Iiyama S, et al. DNMT3a expression pattern and its prognostic value in lung adenocarcinoma. Lung Cancer 2016;97:59-65. [Crossref] [PubMed]
- Bremnes RM, Busund LT, Kilvaer TL, et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:789-800. [Crossref] [PubMed]
- Besser MJ, Shapira-Frommer R, Schachter J. Tumor-Infiltrating Lymphocytes: Clinical Experience. Cancer J 2015;21:465-9. [Crossref] [PubMed]
- Solinas C, Pusole G, Demurtas L, et al. Tumor infiltrating lymphocytes in gastrointestinal tumors: Controversies and future clinical implications. Crit Rev Oncol Hematol 2017;110:106-16. [Crossref] [PubMed]
- Criscitiello C, Esposito A, Trapani D, et al. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat Rev 2016;50:205-7. [Crossref] [PubMed]
- Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011;6:824-33. [Crossref] [PubMed]
- Kataki A, Scheid P, Piet M, et al. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med 2002;140:320-8. [Crossref] [PubMed]
- Andersen R, Donia M, Westergaard MC, et al. Tumor infiltrating lymphocyte therapy for ovarian cancer and renal cell carcinoma. Hum Vaccin Immunother 2015;11:2790-5. [Crossref] [PubMed]
- Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [Crossref] [PubMed]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375-9. [Crossref] [PubMed]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71-8. [Crossref] [PubMed]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol 2002;3:221-7. [Crossref] [PubMed]
- Wang W, Hodkinson P, McLaren F, et al. Histologic assessment of tumor-associated CD45(+) cell numbers is an independent predictor of prognosis in small cell lung cancer. Chest 2013;143:146-51. [Crossref] [PubMed]
- Michie CA, McLean A, Alcock C, et al. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 1992;360:264-5. [Crossref] [PubMed]
- Kryczek I, Grybos M, Dlubek D, et al. Accumulation of CD45RO(+) cells in peritoneal carcinomatous fluid favours survival of ovarian carcinoma patients. Cancer Immunol Immunother 2002;51:513-9. [Crossref] [PubMed]
- Rangel R, Rocha L, Ramirez JL, et al. Generation of memory CD4+, CD8+, CD45RO+ and CD16- lymphocytes activated with IL-2, INF-gamma, and TNF-alpha with specific cytotoxicity against autologous cervical cancer cells in a mixed leukocyte-tumour cell culture. Eur Cytokine Netw 1995;6:195-202. [PubMed]
- Peng RQ, Wu XJ, Ding Y, et al. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stage IIIB colon cancer. BMC Cancer 2010;10:496. [Crossref] [PubMed]
- Yajima R, Yajima T, Fujii T, et al. Tumor-infiltrating CD45RO(+) memory cells are associated with a favorable prognosis breast cancer. Breast Cancer 2016;23:668-74. [Crossref] [PubMed]
- Wang L, Zhai ZW, Ji DB, et al. Prognostic value of CD45RO(+) tumor-infiltrating lymphocytes for locally advanced rectal cancer following 30 Gy/10f neoadjuvant radiotherapy. Int J Colorectal Dis 2015;30:753-60. [Crossref] [PubMed]
- Hotta K, Sho M, Fujimoto K, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer 2011;105:1191-6. [Crossref] [PubMed]
- Osinsky S, Kovelskaya A, Bubnovskaya L, et al. CD8 and CD45RO T lymphocytes in bone marrow of gastric cancer patients: correlation with disseminated tumor cells and disease outcome. Exp Oncol 2015;37:48-52. [PubMed]
- Formica V, Cereda V, di Bari MG, et al. Peripheral CD45RO, PD-1, and TLR4 expression in metastatic colorectal cancer patients treated with bevacizumab, fluorouracil, and irinotecan (FOLFIRI-B). Med Oncol 2013;30:743. [Crossref] [PubMed]


