Novel Asymmetrical Linear Stapler (NALS) for pathologic evaluation of true resection margin tissue
Introduction
Surgical resection is the standard treatment for resectable non-small cell lung cancer (NSCLC). The extent of resection has gradually decreased as studies on surgery for lung cancer have accumulated. In the 1950s, the standard resection for lung cancer was a pneumonectomy. In 1960, Cahan successfully performed a lobectomy with regional lymph node dissection in 48 cases (1). Subsequently, lobectomy was universally accepted and became the standard surgery for lung cancer. However, some patients who had poor pulmonary function required a wedge resection or segmentectomy, i.e., a limited resection, for small peripheral lung cancers (2,3). Limited resection for peripheral early lung cancer was performed not only in patients with poor pulmonary function but also in patients with normal pulmonary function (4,5). In 1995, the Lung Cancer Study Group reported a randomized trial of limited resection for T1N0 stage NSCLC that compared limited resection with a lobectomy, and concluded that locoregional recurrence increased after a limited resection (6). However, this result was confounded by several factors. About 25% of the patients had squamous cell carcinoma and the characteristics of the tumors and distance between the resection margin and tumor were not reported. Consequently, limited resection continued to be studied and performed for early NSCLC, particularly tumors smaller than 2 cm (7-12).
The development of chest computed tomography (CT) resulted in the increased diagnosis of ground glass nodules (GGNs) (13). The GGNs in a thin section in chest CTs are generally correlated with clinical features (14). In 2011, the Japan Clinical Oncology Group reported that the specificity for noninvasiveness is 98.7% in adenocarcinoma ≤2.0 cm with a consolidations/tumor (C/T) ratio ≤0.25 (15). By contrast, a prospective multicenter study reported that pure GGNs and heterogeneous GGNs do not develop into invasive adenocarcinoma within a mean follow-up duration of 4.3 years (16). Based on these findings, limited resection is generally accepted for GGNs ≤2 cm with a C/T ratio ≤0.25.
To decrease locoregional recurrence after a limited resection, it is important to confirm that there are no malignant cells in the resection margin. The conventional triple row stapler does not provide intact resection margin tissue for frozen section biopsy due to the tissue damage caused by the titanium fastener. To examine the pathology, the staples in the resection margin must be removed. However, the resection margin tissues are damaged by the stapler itself in almost all cases, which results in profound difficulty with the pathology examination. To resolve this problem, a stapler that preserves the resection margin is required for more accurate cancer surgery.
In this study, we report a new type of linear stapler that preserves the true resection margin tissue after an organ resection. We evaluated its feasibility and accuracy for assessing true resection margins.
Methods
Novel Asymmetrical Linear Stapler (NALS) for resection margin evaluation
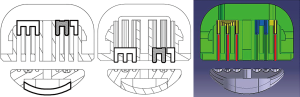
Meditulip (Osong, South Korea) recently developed a NALS for evaluating the resection margin (Figure 1). A conventional linear stapler has two or three symmetrical rows of titanium fasteners on either side of the resection, and a cutter for organ resection between the rows of titanium fasteners. In comparison, NALS has an asymmetrical structure. Figure 2 shows a cross-sectional view of NALS, demonstrating its mechanism. The three rows of titanium fasteners on the remnant organ side are the same as with a conventional linear stapler. These three rows of fasteners close the remnant side tissues securely. In comparison, on the resected organ side, there is a single row of titanium fasteners located farthest from the endo-cutter in the stapler device. With this design, the stapler can provide tissue for a frozen section biopsy at the true resection margin, without the titanium fasteners injuring the tissue. NALS also has a tissue stabilizer (the blue material in Figure 2) that presses the true resection margin tissue to protect the tissue from the forward force of the cutter during firing.
Porcine animal model and operating procedure
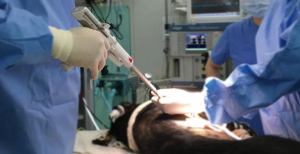
A 6-month-old female Korean native black pig was prepared for the procedure. The animal was quarantined and acclimated in the preclinical animal experiment center in Osong, South Korea, for 1 week before the surgery. With the pig in the left lateral decubitus position, general anesthesia was induced with 2% sevoflurane. A lateral thoracotomy was performed to approach the thoracic cavity. Before performing a wedge resection, the thickness of the porcine lung tissue was measured with calipers, and then the proper site for the wedge resection was determined. Using NALS (a 60-mm long blue stapler with 3.5 mm staples), we performed a wide wedge resection in the right upper lobe to evaluate the stapler. To compare the extent of tissue damage to the true resection margin tissue, we used a popular commercial three-row stapler as a control (Figure 3).
This study was approved by the institutional review board of Osong Medical Innovation Foundation, Cheongju, Chungbuk, Republic of Korea (KBIO-IACUC-2017-004).
Results
The right upper lung was widely resected using both staplers. Both use titanium fasteners of the same height and we used a commercial blue cartridge for medium tissue thickness.
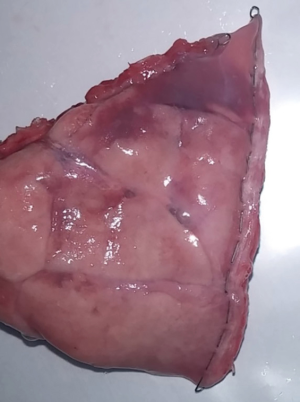
With NALS, we successfully divided and closed the lung tissue (Figure 4). There was no bleeding on either side of the resection margin after the wedge resection. In the saline air-leakage test with 30 cmH2O pressure, there was no air leakage from the remnant organ at the resection margin. The distance between the cutting edge and titanium fasteners was 3.10 mm, allowing a pathological evaluation of the true resection margin. This distance was sufficient to harvest the true resection margin tissue with a scalpel.
With the conventional three-row symmetrical stapler, it was impossible to resect true resection margin tissue without removing the titanium fasteners because the space from the cutting edge to the staples was too narrow. To evaluate the pathology, we had to remove the titanium fasteners and then resect the true resection margin tissue.
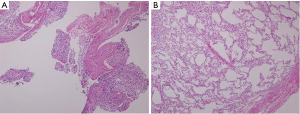
With NALS, there was no squeezing artifact at the true resection margin on microscopic examination and all of the alveolar structures were preserved for evaluation. By contrast, with the conventional stapler, there was severe squeezing artifact and subsequent tissue damage caused by the titanium fasteners making the evaluation extremely difficult (Figure 5).
Discussion
An R0 resection, i.e., a microscopically negative resection margin, is an important factor in oncology surgery. This means that there are no malignant cells remaining in the patient after the operation. For an R0 resection, tissue from the true resection margin must be evaluated during the operation. To do so, a frozen section biopsy can be performed at the true resection margin. In conventional open surgery without a linear staple device, it is easy to obtain fresh undamaged true resection margin tissue. However, when resecting the lung with a linear stapler device, it is impossible to get undamaged true resection margin tissue due to the tissue damage caused by the titanium fasteners. This is very problematic, because it makes it impossible to evaluate the true resection margin. This concern has been raised by both surgeons and pathologists (17). Therefore, this problem needs to be solved to ensure more accurate surgical treatment of cancer patients.
With the development of chest CT, the diagnosis of early NSCLC has improved, which has resulted in an increase in limited resection in patients who can tolerate a lobectomy. The oncological outcomes in recent studies support this trend (18-20). Hence, we are concerned with the failure to obtain an R0 resection with a limited lung resection in early lung cancer.
Previous studies have reported that the distance between the resection margin and the tumor is important for preventing locoregional recurrence (21,22). However, even when the distance between the resection margin and tumor exceeds the tumor size, locoregional recurrence sometimes occurs. To decrease locoregional recurrence, a frozen section biopsy of the true resection margin tissue should be performed to confirm R0 resection. With a conventional stapler, it is difficult to examine the resection margins precisely, due to squeezing artifacts. In comparison, NALS effectively preserves the true resection margin tissue. It is also easy to cut the true resection margin tissue after the stapler is applied, because there is sufficient space to resect the tissue with a scalpel (more than 3 mm). In addition, there is no bleeding from the resected lung fastened with a single row of titanium fasteners during the operation. Therefore, a single row of staples is sufficient for the resected lung side.
A recent study of gastric cancer reported a manually removed modified stapler similar to NALS for evaluation of the true resection margin (23). With that modified stapler, true resection margin tissue could be harvested in gastric cancer without a squeezing artifact. Therefore, this type of linear staple device is useful for evaluating the true resection margin in both lung and solid cancer surgery.
This animal study had several limitations. First, we performed a wide wedge resection in a single pig. Although we demonstrated the feasibility of NALS, we could not determine the statistical difference between it and the conventional stapler. Second, the wedge resection was performed in a pig and not in human tissue. Therefore, more studies of human tissue are required to evaluate potential safety issues.
Conclusions
NALS, which preserves the true resection margin, is useful for evaluating the resection margin with a frozen section biopsy in oncology surgery. Particularly, with a limited resection, such as sublobar resection, in early lung cancer, this stapler device is useful for evaluating the resection margin.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Osong Medical Innovation Foundation, Cheongju, Chungbuk, Republic of Korea (KBIO-IACUC-2017-004).
References
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [PubMed]
- Date H, Andou A, Shimizu N. The value of limited resection for "clinical" stage I peripheral non-small cell lung cancer in poor-risk patients: comparison of limited resection and lobectomy by a computer-assisted matched study. Tumori 1994;80:422-6. [PubMed]
- Miller JI Jr. Limited resection of bronchogenic carcinoma in the patient with impaired pulmonary function. Ann Thorac Surg 1993;56:769-71. [Crossref] [PubMed]
- Ginsberg RJ. Limited resection in the treatment of stage I non-small cell lung cancer; an overview. Chest 1989;96:50S-1S. [Crossref] [PubMed]
- Pastorino U, Valente M, Bedini V, et al. Limited resection for Stage I lung cancer. Eur J Surg Oncol 1991;17:42-6. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Fibla Alfara JJ, Gómez Sebastián G, Farina Ríos C, et al. Lobectomy versus limited resection to treat non-small cell lung cancer in stage I: a study of 78 cases. Arch Bronconeumol 2003;39:217-20. [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [Crossref] [PubMed]
- Nonaka M, Kadokura M, Yamamoto S, et al. Tumor dimension and prognosis in surgically treated lung cancer: for intentional limited resection. Am J Clin Oncol 2003;26:499-503. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Nakamura H, Kazuyuki S, Kawasaki N, et al. History of limited resection for non-small cell lung cancer. Ann Thorac Cardiovasc Surg 2005;11:356-62. [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;129:1226-31. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. The prognosis of invasive adenocarcinoma presenting as ground-glass opacity on chest computed tomography after sublobar resection. J Thorac Dis 2017;9:3782-92. [Crossref] [PubMed]
- Yano M, Yoshida J, Koike T, et al. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;47:135-42. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Tsujimoto H, Tsuda H, Hiraki S, et al. In vivo evaluation of a modified linear stapling device designed to facilitate accurate pathologic examination of the surgical margin. Gastric Cancer 2016;19:666-9. [Crossref] [PubMed]



