Aorta-atria-septum combined incision for aortic valve re-replacement
Introduction
Aortic valve reoperation and annulus enlargement in the patient with previous mitral and aortic valve replacement is technically challenging. We share a case about this operation through a combined cardiac incision.
Case presentation
The patient was a 52-year-old female who was admitted in our center in 2016 with exertional dyspnea and episode of syncope in recent period. She had undergone mitral and aortic valve replacements 10 years ago due to rheumatic heart valve disease. In the previous operation, a bileaflet mechanical aortic valve, CarboMedics sized 19, was implanted with supra-annular technique. Anticoagulation therapy with Warfarin was continuous since first operation, and the INR value was satisfactorily controlled around 2.5. Physical examination revealed over grade 3/6 ejective systolic murmurs at aortic auscultation. Transthoracic echocardiogram confirmed that the mean pressure gradient across the aortic valve was 56 mmHg, with normal leaflet motion of aortic prosthesis, and normal function of mitral prosthesis.
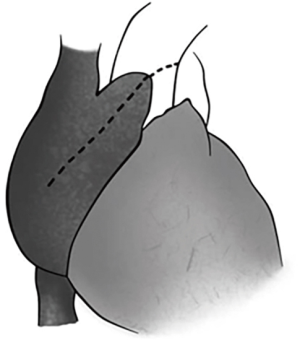
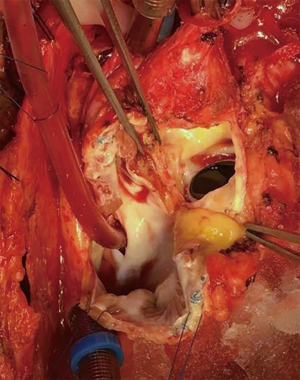
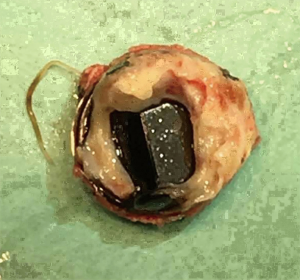
The preoperative evaluation indicated that the minimal size of aortic prosthetic valve is 21 mm based on her body surface area (BSA), to avoid prosthesis patient mismatch (PPM). The patient was warranted redo aortic valve re-replacement and annulus enlargement. After separating the pericardial adhesion, cardiopulmonary bypass was established by ascending aorta cannulation, superior and inferior vena cava. Aorta was cross clamped, and the cold blood cardioplegia solution was delivered through aortic root. Right atrium (RA) was opened after vena cava snaring. Atrial septum was opened and left atrial vent was inserted. An oblique aortotomy on the ascending aorta was made, and extended downward to merge with incisions of RA and upper atrial septum. The aortic annulus was then opened approximately 6–7 mm away from the ostium of right coronary. The commissure between the non-coronary and right coronary sinus was hard to be identified, and subaortic triangular curtain was disappeared. This incision will keep two sides of the aortic root and the RA wall intact (Figure 1). With a satisfactory visual operative field, the prosthetic valve and subvalvular area were easy and carefully assessed. It was found that subvalvular pannus formed circlewise, which reduced the orifice area but did not limit the motion of prosthesis leaflets. The sewing ring and the banding structure of the mechanical prostheses were cut down thoroughly, so that the housing of the valve was easily taken out by pulling with a tonsil clamp. The circumferential pannus on the inflow side was formed with an orifice about 10 mm, which obstructed the left ventricular outlet (LVOT) (Figure 2). The pannus was originated the felt pledgets that placed in the primary operation (Figure 3). The fabric of the sewing ring and the pannus in the LVOT were completely removed with #11 blade. The aortic incision was further extended to the mitral annulus. An autologous pericardial patch treated with glutaraldehyde was trimmed as the spindle shape and proper size guided with #21 valve sizer in place at the aortic position. The aortic annulus was enlarged with the patch and 4-0 Prolene continuous suturing as Nick’s technique. To facilitate the supra-annular valve implantation, multiple mattress sutures with pledget were inserted in the neo-annulus, which part of the pledgets were underneath the native annulus, and part on the right side of the autologous patch. A 21-mm ATS aortic mechanical heart valve was secured and the sutures were tied. The double arm sutures of enlargement were continuous upward along the rims of aortic sinus, and merged at ascending aortic incision to close the aortotomy. Another 4-0 Prolene was used to close the RA. The heart was de-aired, and aorta was unclamped. The clamping time was 82 minutes. The heart re-beated to atrial fibrillation rhythm without electric defibrillation. The patient was weaned off cardiopulmonary bypass with stable hemodynamics. Bleeding control was not difficult and the chest tube drainage in the first day after completion of surgery is 790 mL. Oral anticoagulation therapy with Warfarin was taken to maintain INR 2.0 to 3.0. The postoperative recovery was uneventful. The patient was discharged to home on postoperative day 7. The patient is doing well after 3 months follow-up with New York Heart Association functional class I.
Comments
Prosthetic valve dysfunction is a serious complication after mechanical valve replacement, which can be classified to structural valve dysfunction and non-structural valve dysfunction. Structural valve dysfunction was only seen in first-generation mechanical valves (1-3). The incidence was 0.1–0.24% per patient year (4-6). Non-structural valve dysfunction is caused by thrombosis, thromboembolism, endocarditis, pannus formation and paravalvular leak.
Pannus formation is one of the most common causes to trigger non-structural valve dysfunction. Pannus circlewise formed under the prosthetic annular could obstruct the blood flow of outflow tract, thus increased the transvalvular pressure gradients and lead a series of symptoms. Although pannus formation had not impacted on the movement of mechanical valve leaflet in this case, it had significantly increased the gradience which was warranted reoperation. Maria-Sinziana Moldovan and the colleagues reported a patient whose mechanical aortic prosthesis closure was interfered by sub-annular pannus (7).
In the previous published papers, the epidemiologic features of pannus formation after aortic valve replacement were that women have higher risk to grow pannus than men, and it usually occurred one or two decades after initial surgery (8,9). Some reported that pannus formation causing prosthetic aortic valve stenosis occurred mainly in female patients with a small BSA (10,11). Histologically the pannus is an overgrowth of fibrous tissue which composed by myofibroblasts, endothelial cells, chronic inflammatory cells and an extracellular matrix such as collagen fiber (12). It may be associated with a healing process of periannular tissue via the expression of transforming growth factor beta 1, protein C and protein S (10,13). There is no exact evidence to prove the relationship between pannus formation and the location of felt pledget.
Reoperation is the effective and essential treatment to the patients whose pannus obstruct the left ventricular outflow tract. The majority of these patients were with small aortic annulus and received 17 or 19 mm mechanical valves in the initial procedure. The aortic annulus orifice was further reduced by subvalvular pannus and secondary hyperplastic ventricular septum. The annulus enlargement procedure had to been done concurrently with aortic valve replacement at the second operation to improve hemodynamics and long-term survival. The common approaches include Nicks, Konno and Manouguian techniques. All of these methods need optimal surgical exposure of aorta root, which is usually accompanied by tight adhesion with right atrial appendage and hard to dissect in the second operation. Massive adherence separation may cause injury of RA, aorta, and even right coronary artery. In order to avoid the risky anatomy and make the second procedure simple, we here implied the alternative incisions that extend the oblique aortotomy across the commission of right coronary cusp and the noncoronary cusp, and simultaneously incise the partial RA which adhere to the aorta. After the aortic root has been enlarged sufficiently, a spindle-shaped patch of autologous or prosthetic material is inserted to the incision with continuous suture. Comparing to regular Nick’s procedure, this incision avoid overmuch separation resulting in excellent root exposure, short operation time, and less bleeding complication.
In summary, we report this case to remind the cardiac surgeons to be alert to the pannus formation after mechanical aortic valve implantation, and provide a new feasible incision for the aortic root and valve reoperation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gunn JM, Malmberg M, Vähäsilta T, et al. Thirty-year results after implantation of the Björk-Shiley convexo-concave heart valve prosthesis. Ann Thorac Surg 2014;97:552-6. [Crossref] [PubMed]
- Harrison DC, Ibrahim MA, Weyman AE, et al. Miller DE. The Björk-Shiley convexo-concave heart valve experience from the perspective of the supervisory panel. Am J Cardiol 2013;112:1921-31. [Crossref] [PubMed]
- Shiono M, Sezai Y, Sezai A, et al. Longterm results of the cloth-covered Starr-Edwards ball valve. Ann Thorac Surg 2005;80:204-9. [Crossref] [PubMed]
- Butchart EG, Lewis PA, Grunkemeier GL, et al. Low risk of thrombosis and serious embolic events despite low-intensity anticoagulation. Experience with 1,004 Medtronic Hall valves. Circulation 1988;78:I66-77. [PubMed]
- Esmaeilzadeh M, Mirdamadi A, Parsaee M, et al. Is the Peak-to-Mean Pressure Gradient Ratio Useful for Assessment of Aortic Valve Prosthesis Obstruction? J Tehran Heart Cent 2010;5:69-73. [PubMed]
- Rizzoli G, Guglielmi C, Toscano G, et al. Reoperations for acute prosthetic thrombosis and pannus: an assessment of rates, relationship and risk. Eur J Cardiothorac Surg 1999;16:74-80. [Crossref] [PubMed]
- Moldovan MS, Daniela B, Emese K, et al. Pannus-related Prosthetic Valve Dysfuncion. Clujul Medical 2016;89:169-75. [Crossref] [PubMed]
- Sakamoto Y, Hashimoto K, Okuyama H, et al. Prevalence of pannus formation after aortic valve replacement: clinical aspects and surgical management. J Artif Organs 2006;9:199-202. [Crossref] [PubMed]
- Barbetseas J, Nagueh SF, Pitsavos C, et al. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol 1998;32:1410-7. [Crossref] [PubMed]
- Teshima H, Fukunaga S, Takaseya T, et al. Obstruction of St. Jude medical valves in the aortic position: plasma transforming growth factor type beta 1 in patients with pannus overgrowth. Artif Organs 2010;34:210-5. [Crossref] [PubMed]
- Darwazah AK. Recurrent pannus formation causing prosthetic aortic valve dysfunction: is excision without valve re-replacement applicable? J Cardiothorac Surg 2012;7:62. [Crossref] [PubMed]
- Teshima H, Hayashida N, Yano H, et al. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg 2003;126:401-7. [Crossref] [PubMed]
- Ohata T, Sakakibara T, Takano H, et al. Acute thrombotic obstruction of mitral valve prosthesis: low protein C level. Asian Cardiovasc Thorac Ann 2002;10:165-6. [Crossref] [PubMed]