Early experience with transcatheter mitral valve replacement: successes, challenges, and future directions
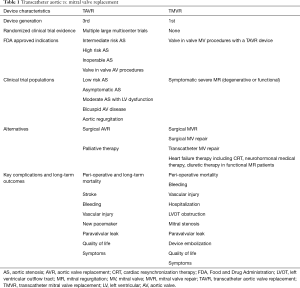
Over the past decade, transcatheter aortic valve replacement (TAVR) has revolutionized the treatment of patients with severe aortic stenosis (AS). TAVR gained a foothold as a viable aortic valve replacement (AVR) strategy in patients deemed inoperable, and early clinical trials in this setting confirmed a clear and dramatic survival advantage of TAVR over palliative medical therapy (1). In the ensuing years, TAVR has provided a paradigm shift in AS treatment and is now an established alternative to surgical AVR in AS patients with ≥ intermediate risk (2,3). Furthermore, TAVR is currently under clinical trial testing in low risk and asymptomatic AS patients (4). In contrast, the development and adoption of transcatheter mitral valve replacement (TMVR) devices has been slower and with its own set of unique challenges. In 2018, TMVR is not approved by the United States Food and Drug Administration (FDA) for patients with native mitral valve disease, and 1st generation devices remain in clinical trial testing in high or extreme risk patients with severe symptomatic mitral regurgitation (MR) (Table 1).
Full table
In 2009, the first TMVR procedure in a human to our knowledge was performed as a trans-apical valve-in-valve procedure in a patient with a stenotic bioprosthetic mitral valve (5). The patient unfortunately died 47 days later due to complications of a postoperative stroke. However, in the ensuing decade considerable progress has been made in advancing the field of TMVR and valve-in-valve TMVR is now a well-established and FDA approved therapy (6-9). However, in contrast to valve-in-valve procedures, native valve TMVR remains in the infancy of clinical trial testing due to a number of challenges including lack of a rigid annulus, subvalvular anatomic complexity, and risk of device embolization (10). In 2013, the first reported cases of native valve TMVR in humans were performed in Europe using trans-apical approach in patients with severe mitral annular calcification (MAC) and mitral stenosis (11,12). In recent years native valve TMVR in patients with primary MR has emerged with the use of devices specifically designed for the mitral position with technology to facilitate anchoring in a non-calcified, non-rigid annulus (13-18). Early results of the Tendyne Valve (Abbott, Santa Clara, CA, USA) in 30 patients with severe native MR at high risk for surgery (mean Society of Thoracic Surgeons Predicted Risk of Mortality or STS PROM 7.3%) were encouraging with 87% of patients having successful device implantation free of cardiovascular mortality, stroke, or device malfunction at 30 days (17).
In a recent edition of the Journal of the American College of Cardiology, Bapat et al. report their findings from a prospective single-arm trial of the Intrepid Valve (Medtronic, Minneapolis, MN, USA), a novel self-expanding bovine pericardial TMVR device for native valve MR (19). In this trial of the first 50 consecutive patients referred for treatment with the device, the authors report the following key findings. First, this was an elderly (mean age 73±9 years), highly symptomatic (86% ≥ New York Heart Association or NYHA class 3), frail (frailty 32%, low albumin 23%, anemia 44%) population with high rates of major comorbidities (58% chronic renal insufficiency, 44% prior sternotomy, 30% malignancy). The majority of patients had functional MR (84%) and the STS PROM was 6.4%±5.5%. Second, the rates of 30-day mortality (14%), bleeding (18%), and re-operation (10%) were high. Third, the technical results of the procedure were excellent with 48/50 (96%) patients having technically successful device implantation. At 30 days, all 42 living patients had either no MR or mild MR by echo, and there were no cases of left ventricular outflow tract (LVOT) obstruction due to device implantation and no cases of device embolization. Additionally, among 42 living patients NYHA class significantly improved at 30 days, and among 13 reported patients the Minnesota Living With Heart Failure Questionnaire scores significantly improved at 12 months.
The development of safe and effective TMVR devices faces certain unique challenges distinct from those of TAVR devices. The study by Bapat et al. provides positive steps forward in TMVR on several fronts. First, the anatomy of the mitral valve is more complex and multi-faceted than the aortic valve anatomy. The mitral valve has a saddle shaped annulus which can dilate under conditions of left ventricular dysfunction. Moreover, the mitral valve apparatus includes not only the annulus and leaflets, but also the subvalvular apparatus including chords and papillary muscles and the left ventricular myocardium itself. The complexity of mitral anatomy and its propensity to distort under conditions of pathology lends to challenges in ensuring adequate device fixation for transcatheter heart valves. For this reason, early successes in the TMVR arena have been achieved in valve-in-valve (9) or valve-in-MAC (11,12) procedures which provide a more suitable fixation platform than the native non-calcified mitral annulus. However, the Intrepid Valve uses an outer frame with flexible atrial portion and cleats which allows the device to conform to the annular anatomy and to provide sites of friction for valve fixation. The results are impressive with no cases of device embolization or significant paravalvular leak. The second issue unique to mitral anatomy is the close proximity to the LVOT. In patients with small left ventricles, the close relationship of the anterior mitral leaflet and LVOT may lead to significant LVOT obstruction with TMVR implantation. In the series by Bapat et al. only patients with a projected LVOT area of 1.3 cm2 post valve implantation were included, and the authors report no cases of significant LVOT obstruction. These findings are especially encouraging in a series that included 42% women in whom smaller left ventricular size may contribute to higher risk of LVOT encroachment. Third, the authors report impressive improvements in both functional status and quality of life in this early experience with the Intrepid Valve. These findings are especially encouraging when considering the very high proportion of functional MR patients in whom these endpoints are critically important. There is no randomized trial evidence to support a mortality benefit of mitral valve replacement. Therefore, proving that TMVR offers improved functional capacity and quality of life are key aspects on the path to eventual adoption of TMVR in routine clinical practice.
However, considerable work remains before TMVR can be adopted with the same excitement and promise of TAVR. The findings by Bapat et al. highlight a number of key issues which will require further attention in coming years. First, the 30-day mortality rate of 14% in this series remains alarmingly high. Considering that the predicted risk of surgical mortality was 6.4% in this cohort of patients felt to be of inoperable risk, strategies to reduce TMVR related mortality will be critically important. Of the 7 deaths in this series, 3 (6% of the study population) were due to access site bleeding. An additional patient did not undergo TMVR due to access site bleeding and 5 patients (10%) underwent re-operation for bleeding complications. The overall rate of major bleeding was 18%. The high rates of bleeding-related mortality and bleeding-related reoperation highlight the major procedural risks in this older frail population with requisite need for post-procedural anticoagulation who are treated with a thoracotomy and left ventricular apical access. Future development of safe and effective transfemoral transseptal approaches to TMVR should obviate the need for thoracotomy and left ventricular apical access and offer the potential for lower bleeding related complications. Moreover, further study of the ideal post-procedure anticoagulant regimen and duration will be of utmost importance in optimizing peri-procedural safety and potentially long-term valve performance and hemodynamics.
As the field of TMVR continues to evolve, several unknowns remain. Unlike TAVR in which the alternative strategies are clear (either surgical AVR or palliation), there are a wide range of therapies for severe MR. For patients in whom surgery is high or extreme risk, a number of transcatheter mitral repair options may be considered. The edge to edge repair MitraClip device (Abbott, Santa Clara, CA, USA) is the predominant transcatheter mitral repair device currently in use (20,21), but a number of other devices are in development including a variety of annuloplasty devices (22). Early experience with commercial use of the MitraClip in >500 patients in the United States is very encouraging with 91% procedural success and the majority of patients discharged to home with moderate or less MR (23). It is unknown whether transcatheter mitral repair or replacement will be superior in patients eligible for either approach (24). Additionally, the prognosis of severe MR is variable and depends on patient comorbidities, left ventricular function, and other factors. Therefore, patients with severe functional MR may be treated with optimal heart failure medical therapies or cardiac resynchronization therapy in eligible patients resulting in improvements in MR severity and symptoms (25). Unlike severe AS, in which medical therapy is strictly palliative, medical therapy and cardiac resynchronization in functional MR patients may impact the severity and course of disease. Therefore, patient selection will likely remain paramount to all decisions in the clinical use of TMVR technology. A heart team approach involving interventional and imaging cardiologists and cardiac surgeons among others will be necessary to identify patients most likely to benefit from TMVR. Shared decision making between patients and providers is also likely to have a critical role in this process.
In conclusion, the rapid evolution of transcatheter solutions for patients with valvular heart disease continues in the arena of native valve MR with emerging devices for both mitral repair and replacement. Lessons learned from TAVR and mitral valve-in-valve procedures, including use of a heart team for ideal patient selection, will be critical in achieving further success in TMVR development in the coming years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218-25. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Vahl TP, Kodali SK, Leon MB. Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure. J Am Coll Cardiol 2016;67:1472-87. [Crossref] [PubMed]
- Cheung A, Webb JG, Wong DR, et al. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann Thorac Surg 2009;87:e18-20. [Crossref] [PubMed]
- Cheung AW, Gurvitch R, Ye J, et al. Transcatheter transapical mitral valve-in-valve implantations for a failed bioprosthesis: a case series. J Thorac Cardiovasc Surg 2011;141:711-5. [Crossref] [PubMed]
- Dvir D, Assali A, Vaknin-Assa H, et al. Transcatheter aortic and mitral valve implantations for failed bioprosthetic heart valves. J Invasive Cardiol 2011;23:377-81. [PubMed]
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012;5:341-9. [Crossref] [PubMed]
- Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol 2013;61:1759-66. [Crossref] [PubMed]
- Van Mieghem NM, Piazza N, Anderson RH, et al. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol 2010;56:617-26. [Crossref] [PubMed]
- Hasan R, Mahadevan VS, Schneider H, et al. First in human transapical implantation of an inverted transcatheter aortic valve prosthesis to treat native mitral valve stenosis. Circulation 2013;128:e74-6. [Crossref] [PubMed]
- Sinning JM, Mellert F, Schiller W, et al. Transcatheter mitral valve replacement using a balloon-expandable prosthesis in a patient with calcified native mitral valve stenosis. Eur Heart J 2013;34:2609. [Crossref] [PubMed]
- Cheung A, Webb J, Verheye S, et al. Short-term results of transapical transcatheter mitral valve implantation for mitral regurgitation. J Am Coll Cardiol 2014;64:1814-9. [Crossref] [PubMed]
- Cheung A, Stub D, Moss R, et al. Transcatheter mitral valve implantation with Tiara bioprosthesis. EuroIntervention 2014;10 Suppl U:U115-9.
- De Backer O, Piazza N, Banai S, et al. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv 2014;7:400-9. [Crossref] [PubMed]
- Quarto C, Davies S, Duncan A, et al. Transcatheter Mitral Valve Implantation: 30-day Outcome of First-in-Man Experience With an Apically Tethered Device. Innovations (Phila) 2016;11:174-8. [Crossref] [PubMed]
- Muller DWM, Farivar RS, Jansz P, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial Experience of Transcatheter Mitral Valve Replacement With a Novel Transcatheter Mitral Valve: Procedural and 6-Month Follow-Up Results. J Am Coll Cardiol 2015;66:1011-9. [Crossref] [PubMed]
- Bapat V, Rajagopal V, Meduri C, et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12-21. [Crossref] [PubMed]
- Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013;62:317-28. [Crossref] [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Feldman T, Guerrero M. Transcatheter direct mitral valve annuloplasty: a brief review. EuroIntervention 2015;11 Suppl W:W53-7.
- Sorajja P, Mack M, Vemulapalli S, et al. Initial Experience With Commercial Transcatheter Mitral Valve Repair in the United States. J Am Coll Cardiol 2016;67:1129-40. [Crossref] [PubMed]
- Maisano F, Alfieri O, Banai S, et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. replacement? Eur Heart J 2015;36:1651-9. [Crossref] [PubMed]
- van Bommel RJ, Marsan NA, Delgado V, et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation 2011;124:912-9. [Crossref] [PubMed]


