Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices—5-year follow-up
Introduction
Left ventricular assist device (LVAD) implantation has become an established treatment option for patients with end stage heart failure. It is either used as bridge to transplantation or as destination therapy. All current devices still require an external power source with energy supplied via a tunneled percutaneous driveline. Driveline infections (DLI) are one of the leading causes for unplanned re-admission of patients with LVAD (1,2). Considerable efforts are undertaken to minimize DLI rates and surgical trauma, for example by the use of anchoring devices (3), externalization techniques (4) or minimally invasive techniques (5-8).
In 2011 our group investigated the parameters of the patients who had received LVAD-implantation in the years 2008 and 2009 using a conventional tunneling technique (group 1) and compared them with those, who started LVAD-therapy in 2011 (group 2). This analysis was inspired by the fact that we changed our usual implantation method to a double tunneling technique through the musculus rectus abdominis, providing a longer intrafascial run in 2011 (9). The data, collected 1 year after the implantation, showed that the new implantation technique markedly reduced DLI rate. However, overall mortality showed no significant improvement. In this study, we follow up on the aforementioned cohort and present outcomes as well as infection rates of a single center cohort, 5 years after implementing the double tunnel technique for driveline implantation.
Methods
Patients
We retrospectively analyzed the data of all the patients, who received a HeartWare ventricular assist device (HVAD) (Medtronic, Minnesota, MN, USA) as a ventricular assist device (VAD) therapy in our clinic in the years 2008 and 2009 (group 1, n=36, single tunneling of driveline) versus patients in the year 2011 (group 2, n=33, double tunneling of driveline). Patients on LVAD support less than 40 days were excluded from the study cohorts. The database of a single center was used for the performance of this retrospective study. All causes of death and adverse events were determined by retrospective examination of medical records.
Surgical procedure
LVAD implantation was performed using a standardized procedure, as per manufacturer’s instructions, with access either via a full sternotomy or via a partial upper hemisternotomy combined with anterolateral thoracotomy. Patients in group 1 were operated by the conventional driveline tunnel method with the driveline exiting directly through the abdominal wall. The double tunnel driveline technique includes placement of the driveline in the sheath of the rectus muscle in the umbilical direction and then subcutaneously to the left upper quadrant (group 2). Before the implantation the driveline itself was dipped in antibiotic. A pre- and postoperative antibiotic prophylaxis (up to 7–10 days postoperative) was consistently administered to all patients. In order to allow anchoring of the driveline exit site, manipulation was avoided for at least 10 days.
Follow up
All patients were monitored regularly in our outpatient clinic. In case of erythema detection at the driveline exit site, patients were treated with regular wound dressings, mechanical lavage and oral antibiotics depending on microbiological cultures. In case of ongoing infection patients were admitted to the hospital and treated with intravenous antibiotics, surgical debridement and vacuum-therapy if necessary. In the event of treatment failure patients were put on the high urgency list for heart transplantation. Upon occurrence of an ascending infection LVAD exchange was performed.
Statistical analysis
SPSS 23.0 package (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data are presented as mean ± standard deviation. The unpaired t-test was applied to compare the incidence of DLI between group 1 and 2 with statistical significance considered for P values <0.05. We also used unpaired t-test with P value <0.05 to identify variables associated with the occurrence of DLI. Kaplan-Meier analysis with survival distribution function was performed to assess how far the survival was prolonged. Log-rank, Wilcoxon and Tarone-Ware test with P value <0.05 were made to check equality of the survival distribution functions.
Results
Baseline characteristics
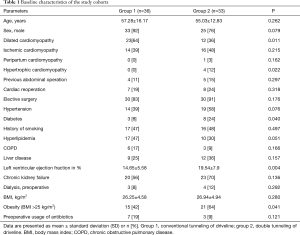
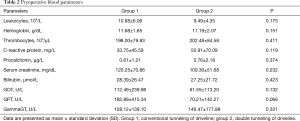
The baseline characteristics are depicted in Table 1. To begin with, both study groups show comparable baseline characteristics. Group 2 included more patients diagnosed with diabetes and high BMIs. Preoperative left ventricular ejection fraction was lower in patients of group 1. There was also a difference in the underlying disease etiology with a higher rate of dilated cardiomyopathy in group 1. Preoperative laboratory parameters of the two groups were also similar and are shown in Table 2.
Full table
Full table
Surgical implantation time was considerably longer in group 2 (158.14±47.88 min in group 1 vs. 202.84±56.03 min in group 2, P<0.001, Table 3). We could not detect substantial differences between the two cohorts regarding the postoperative course. However, the frequency of reoperations was reduced in group 2, although without statistical significance [53% (n=19) group 1 vs. 33% (n=11) group 2, P=0.053, Table 4]. The 5-year mortality was lower in group 2 [42% (n=15) in group 1 vs. 27% (n=9) in group 2] but was not statistically significant (P=0.107). The days of support between the two groups were comparable (1,275.56±885.89 in group 1 vs. 1,321.94±711.37 in group 2, Table 4).

Full table

Full table
Infection rate
The rate of DLI in the double tunnel group (group 2) was significantly lower than in the conventional tunnel group (group 1) [61% (n=22) group 1 vs. 30% (n=10) group 2, P=0.005] and almost identical to the 5-year infection rate, since only one patient developed his first DLI after 5 years of LVAD support (Table 5). As well group 1 had significantly more stationary admissions and needed more conservative therapy for their DLI than in group 2 (Table 5).

Full table
Most of the positive microbiological results of the driveline smears showed presence of staphylococcus aureus (9 of 32). Several other microbial infections were also detected, including staphylococcus epidermidis, corynebacterium spp. and proteus mirabilis.
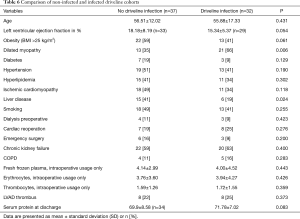
Furthermore new cohorts were created for further investigation. Patients with and without DLI were arranged into groups 1 and 2 respectively. Three patients from group 1 showed a restitution of heart function and the LVAD device was therefore explanted on postoperative day (POD) 478, 996 and 1831. Five patients from group 1 needed an LVAD exchange between 2011 and 2012 due to a device-thrombus on POD 1,641, 886, 675, 790 and 1,017 and were therefore re-implanted by means of the new double tunneling technique. Elevated variables were liver disease (41% of group 1 vs. 19% of group 2, P<0.024) and dilated cardiomyopathy (35% of group 1 vs. 66% of group 2, P<0.006) as underlying diseases. In contrast, serum protein levels did not show a difference between infected and non-infected patients (Table 6).
Full table
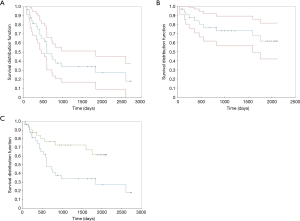
We noticed that the median survival time was lower in the double tunneling group (1,583.027±148.815 days) than in the conventional tunneling group (1,153.495±185.319 days). The comparison of the two survival distribution functions (1,153.495±185.319 days, group 1 vs. 1,583.027±148.815 days, group 2) led us to the conclusion that double tunneling method had significantly improved the survival time of patients (Table 7, Figure 1).

Full table
Discussion
LVAD-specific infections still remain a major challenge in LVAD therapy (10). With the help of this long term follow up study we were able to show that the double tunnel technique leads to a significant reduction of DLI in patients on LVAD support 5 years after the implantation. These results are consistent with the results of the group observed for 1 year after the LVAD implantation (9). Dean et al. as well as Schibilsky et al. have also recently detected the conventional driveline implantation technique to be an important risk factor for DLI (11,12). The double tunnel technique results in a longer, intrafascial run, which creates a large barrier for ascending infections and causes less driveline trauma by improving driveline stabilization. With the current study and a follow up time of 5 years we managed to adjust LVAD support days and rule out confounding effects.
Furthermore our study revealed that dilated cardiomyopathy as underlying disease was associated with higher infection rate, although there are no studies to confirm these results in the medical literature. However, distribution of cardiomyopathy differed significantly between the two groups, acting as a possible confounder.
Five patients from group 1 received an LVAD exchange via the double tunneling technique during the 5-year observation period, possibly confounding the mortality statistic. We suggest that mortality would be significantly reduced by double tunneling technique if analyzed in a bigger patient collective.
Recently Imamura et al published data on a score using serum albumin and body mass index (BMI) predicting DLI (13). In our study low BMI also showed an association, although not statistically significant, with a higher infection rate. Since serum albumin was not measured in our patients, we compared serum protein levels, but could not detect a difference between infected and non-infected patients. Existing literature suggests young age to be another relevant risk factor for DLI (1,14). The current study revealed no correlation between age and infection rates in both groups, possibly due to a small number of patients under investigation.
DLI are the most frequent complications in LVAD patients. Their treatment leads to recurrent hospital re-admission which strongly affects patients’ quality of life. Dealing with chronic DLI can be complicated. If antibiotic therapy fails, an exchange of the LVAD remains the inevitable alternative (15-17). Altogether using a double tunneling technique for driveline implantation seems to be an important factor to lower infection rates in LVAD patients.
However, further research in bigger populations is required to reveal the effect of double tunneling technique on patient survival.
Limitations
One of the limitations to be considered is predetermined by the retrospective collection of data and therefore it is subject to the limitations associated with retrospective studies. Since all VAD implantations were performed at one center, conclusions may be restricted to institutional experience only. Furthermore the study population is rather small. Larger, prospective, randomized controlled studies might be worthwhile to compare the long-term effects of different driveline tunneling techniques.
Conclusions
The double tunneling technique significantly lowers the risk of DLI 5 years after LVAD implantation.
Acknowledgements
Funding: This manuscript was kindly supported by a grant of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) through the Project “KFO 311” (Principal Investigators: Prof. J Bauersachs, Prof. M Hoeper, Prof. JD Schmitto).
Footnote
Conflicts of Interest: JD Schmitto and G Dogan are consultants of Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethical committee of the Hannover Medical School. Informed consent was obtained from all patients prior to data analysis.
References
- Leuck AM. Left ventricular assist device driveline infections: recent advances and future goals. J Thorac Dis 2015;7:2151-7. [PubMed]
- Akhter SA, Badami A, Murray M, et al. Hospital Readmissions After Continuous-Flow Left Ventricular Assist Device Implantation: Incidence, Causes, and Cost Analysis. Ann Thorac Surg 2015;100:884-9. [Crossref] [PubMed]
- Cagliostro B, Levin AP, Fried J, et al. Continuous-flow left ventricular assist devices and usefulness of a standardized strategy to reduce drive-line infections. J Heart Lung Transplant 2016;35:108-14. [Crossref] [PubMed]
- Singh A, Russo MJ, Valeroso TB, et al. Modified HeartMate II driveline externalization technique significantly decreases incidence of infection and improves long-term survival. ASAIO J 2014;60:613-6. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Schmitto JD, Mohr FW, Cohn LH. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Curr Opin Cardiol 2011;26:118-22. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Past, present, and future of minimally invasive mitral valve surgery. J Heart Valve Dis 2011;20:493-8. [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Fleissner F, Avsar M, Malehsa D, et al. Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices. Artif Organs 2013;37:102-7. [Crossref] [PubMed]
- Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 2011;30:375-84. [Crossref] [PubMed]
- Dean D, Kallel F, Ewald GA, et al. Reduction in driveline infection rates: Results from the HeartMate II Multicenter Driveline Silicone Skin Interface (SSI) Registry. J Heart Lung Transplant 2015;34:781-9. [Crossref] [PubMed]
- Schibilsky D, Benk C, Haller C, et al. Double tunnel technique for the LVAD driveline: improved management regarding driveline infections. J Artif Organs 2012;15:44-8. [Crossref] [PubMed]
- Imamura T, Kinugawa K, Nitta D, et al. Readmission due to driveline infection can be predicted by new score by using serum albumin and body mass index during long-term left ventricular assist device support. J Artif Organs 2015;18:120-7. [Crossref] [PubMed]
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [Crossref] [PubMed]
- Hanke JS, Haverich A, Schmitto JD. Exchange of a HeartMate II left ventricular assist device with a HeartMate 3 pump. J Heart Lung Transplant 2016;35:944-6. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Dogan G, et al. First series of left ventricular assist device exchanges to HeartMate 3. Eur J Cardiothorac Surg 2017;51:887-92. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]


