Cardiac radiation dose distribution, cardiac events and mortality in early-stage lung cancer treated with stereotactic body radiation therapy (SBRT)
Introduction
Radiation dose to the heart has been associated with cardiovascular mortality and cardiac complications predominantly in long term survivors with breast cancer (1-3), Hodgkin’s lymphoma (4) and pediatric malignancies (5). Cardiac toxicity appears to be dependent on the radiation dose and radiation technique (1,6,7). Radiation therapy dose to various cardiac substructures has been associated with coronary artery, pericardial, and conduction system diseases as well as cardiomyopathy (8). For breast cancer, the radiation dose distribution to various heart regions has been well-described (9). In addition, a recent study demonstrated a clinically-relevant dose-effect relationship between mean heart dose (MHD), left ventricle volume receiving 5 Gy and acute coronary events (6).
Recently, interest in cardiac toxicity in lung cancer patients has increased (10). Several studies showed that radiation dose to the heart in stage III lung cancer patients was associated with the development of cardiac events (7,11-15) and overall survival (12,14,15). Importantly, RTOG 0617 showed that the heart volume receiving ≥5 and 30 Gy predicted overall survival. While cardiac toxicity is usually a late event, cardiotoxicity has also been observed early after radiotherapy (11). Because of this, it is important to investigate earlier time points in addition to the late ones. Cardiotoxicity in patients treated with SBRT for early-stage lung cancer delivering higher doses per fraction to smaller subvolumes depending on the individual tumor location has not been studied in great detail. In this setting, patients who are inoperable or who choose not to undergo surgery are treated with radiation therapy. Patients with early-stage lung cancer have a better prognosis than locally-advanced lung cancer patients (16) and thus are more likely to survive to experience cardiac toxicity. Still, despite good locoregional tumor control and a moderate prevalence of distant metastases, 5-year survival rates of 41.2% are surprisingly low indicating that factors other than tumor contribute to high mortality rates (17). Two retrospective studies recently investigated cardiac dose as a predictor of mortality in early-stage lung cancer stereotactic body radiation therapy (SBRT). While one study found no relation between mean dose for the whole heart and survival (18), the other, larger study observed that radiotherapy doses mainly to the upper regions of the heart were associated with non-cancer death (19). Our study aims to provide a more detailed analysis than what is currently available on cardiac dose and patient outcomes after SBRT. We investigate in particular the association of radiation therapy doses to the heart and its multiple substructures with cardiac events and overall survival. In addition, we also analyze the influence of preexisting cardiac disease and other comorbidities with respect to the development of cardiac events and survival.
Methods
Aim, design and setting
Seventy-four patients with 75 tumors treated definitively with SBRT for stage I or II non-small cell lung cancer (NSCLC) (72 cases) and small cell lung cancer (SCLC) (2 cases) at our institution were retrospectively analyzed on an IRB-approved protocol (VCU IRB HM15356). Information was gathered from electronic medical records. Following radiotherapy, patients returned for regular follow-up visits that included clinical history, physical exam and computed tomography imaging every 3 months for the first 2 years, then every 6 months up to 5 years, and yearly afterwards. Patients were seen by cardiologists as clinically indicated. All available medical records from all specialties were utilized for this study. The relation between radiation doses to the heart and cardiac substructures, tumor location, and preexisting medical conditions with the development of cardiac events and mortality was assessed. Any new cardiac conditions that occurred after radiotherapy were recorded as cardiac events. These included diseases of the cardiac conduction system, structural heart disease, heart failure and ischemic cardiac disease.
Patient characteristics
Patients were treated definitively with lung SBRT between 2007 and 2017. All of these patients were medical inoperable or refused surgery. Chemotherapy or surgery were not utilized. Seventy-seven percent of patients received 48 Gy in 4 fractions. Other doses ranged from 40 Gy in 4 fractions to 60 Gy in 8 fractions. The average follow-up was 35 months, median was 27 months, ranging from 1 month to 10 years and 10 months. Sixty-one (82%) of patients were treated for newly diagnosed lung cancer and 13 (18%) were treated for lung cancer recurrence. All recurrences were treated initially with surgery except for three cases which were treated with radiochemotherapy. All patients who underwent thoracic radiation therapy prior to SBRT of lung cancer had negligible heart doses. Details of patient characteristics including preexisting cardiac disease and comorbidities are described in Table 1. Note that all patients with hyperlipidemia, diabetes and hypertension were included in the studies whether the diseases were medically controlled or not.
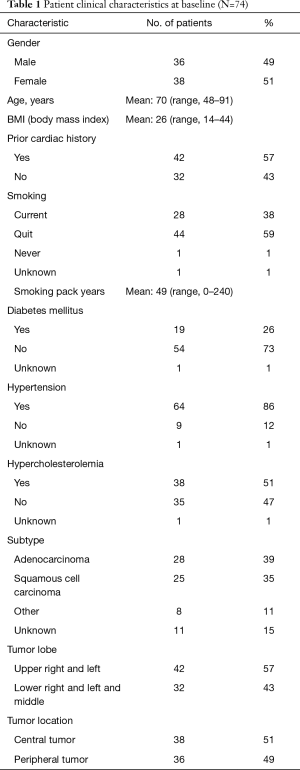
Full table
Further treatment information is listed in Table 2 and details of cardiac conditions are listed in Table 3. Patients were included in this analysis if they had a tumor located within 6 cm of the heart border, as larger distances resulted in minimal (<0.1 Gy) radiation exposure of the heart. The distance was recorded as the smallest distance in cm between the planning target volume (PTV) and heart border. If there was overlap between PTV and heart, then a negative distance was recorded. Central tumor location was defined as being within 2 cm of the mediastinum and central airways.
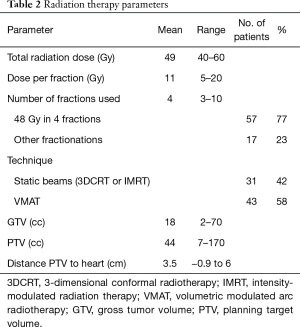
Full table
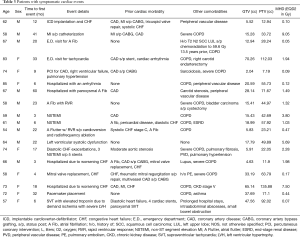
Full table
Dosimetric parameters
The whole heart and cardiac substructures (13 substructures including 2 atria, 2 ventricles, 4 heart valves, AV node and four major coronary artery branches) were contoured on the 30% phase of the 4D treatment planning CT of each patient using commercial treatment planning software Pinnacle (Philips, The Netherlands) and MIM 6.6.10 (Cleveland, OH, USA) according to contouring atlas guidelines (20). For each structure, dose-volume parameters including mean dose, highest dose to 0.03 cm3 (D0.03cc) and percentage of a given structure receiving either ≥5, 10, 20, 30, 40, 50 or 60 Gy (V5-Gy, V10-Gy …) were recorded. D0.03cc was used in accordance with AAPM TG 101 guidelines for SBRT (21). Equivalent doses of 2-Gy fractions (EQD2) were calculated from the dose-volume histograms as nd[(d+α/β)÷(2+α/β)], where n was the number of fractions, d was the dose to the structure per fraction (in Gy), and α/β was 2 Gy (22). For the patient with two tumors treated at the same time, the composite treatment plan was used to calculate the total dose to the heart structures from both treatments.
Statistical analysis
Mann-Whitney U test was used to test significance of differences in cardiac radiation dose for tumor location, treatment technique, cardiac toxicity, and overall and non-cancer survival as well as the difference in survival and cardiotoxicity in patients based on the number of smoking pack years. Pearson correlation coefficient was used to assess the relationship between tumor distance and PTV size with heart radiation dose. Chi-squared distribution and Kaplan-Meier estimator were calculated to determine the relationship between prior cardiac events, diabetes mellitus, hypertension, hypercholesterolemia and cardiotoxicity development and overall survival. All tests were done with a significance level of P<0.05.
Results
Dosimetry
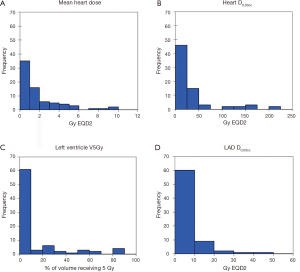
Radiation dose distributions to various cardiac substructures are described in Table 4. All reported doses are expressed in Gy EQD2. There was considerable variability in dose to different cardiac substructures: MHD averaged 1.90 Gy (range, 0.04–11.0 Gy) and average D0.03cc to the left anterior descending artery (LAD) was 5.67 Gy (range 0.04-48.8 Gy). Figure 1A,B,C,D illustrates some of the important dose distributions. Notably, MHD >2 Gy was found in 23 patients and MHD >5 Gy was observed in 8 patients. Ten patients received a D0.03cc of 60 Gy or more. Patients with tumors in the upper lobes received higher MHD and heart D0.03cc compared to the other lobes (3.31 Gy versus 1.15 Gy, P<0.0001 and 41.06 Gy versus 29.51 Gy, P=0.00034). We did not observe a significant difference in MHD or heart D0.03cc between central and peripheral location of the tumors (P=0.84 and P=0.27). The distance between heart and tumor was weakly associated with the MHD (r=−0.61 and P≤0.0001). The magnitude of PTV was only weakly associated with MHD (r=0.41, P=0.0004). Static beams (3DCRT or IMRT) radiation technique did not significantly differ from VMAT for MHD or Heart D0.03cc (P=0.61 and P=0.95). RTOG 0813 constraints (Heart/Pericardium <15 cc max dose is 32 Gy and maximum point dose is 105% of PTV prescription) (23) were met by all patients except for four cases. Of these, only one developed a cardiac event (heart attack) after radiation treatment. In addition, no patient with a distance of >5 cm between PTV edge and heart in this cohort had a MHD >5 Gy.
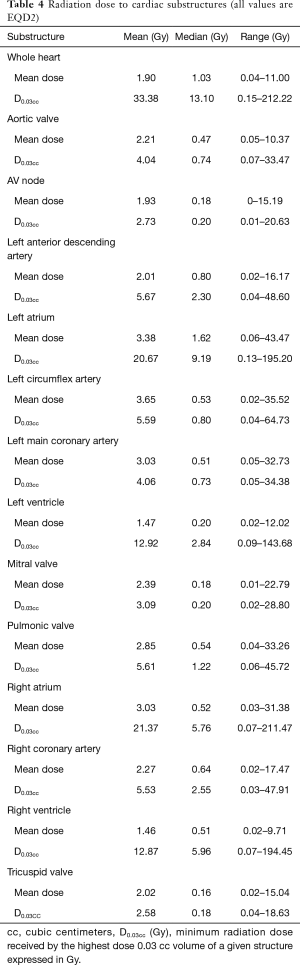
Full table
Mortality
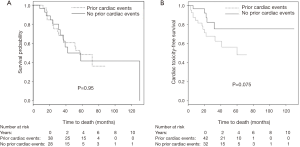
At the time of analysis, 28 patients had died (38%), 20 of non-cancer related deaths (one of them of heart failure) and 8 of cancer progression. 38 were alive (51%), 8 were lost to follow-up. Kaplan-Meier estimated 1-year overall survival was 96%, and 2-year survival was 77%. Prior cardiac disease, radiation dose to the whole heart or the cardiac substructures, number of pack years smoked, diabetes, COPD, hypercholesterolemia and hypertension were not associated with overall mortality (Figure 2A).
Cardiac events
18 patients developed cardiac events after on average 19 months (range, 3–60 months). The cohorts that developed cardiac events and those that did not, had comparable demographics. The most common events were arrhythmia development (8 cases), coronary artery disease (7 cases), congestive heart failure (5 cases) and one case of mitral valve replacement (in addition to a worsening heart failure). Details of cardiac events are listed in Table 3. While arrhythmias occurred on average at 26 months, coronary artery disease and heart failure occurred on average at 15 and 13 months, respectively. Prior cardiac disease was associated with an increased rate of cardiac events after radiotherapy (14 of 42 patients with prior cardiac history developed cardiac events compared to 4 out of 32 patients with no prior history of cardiac events (odds ratio =3.5, P=0.039). Cardiac event-free survival was associated with prior cardiac disease (P=0.075, Figure 2B). Radiation dose to the whole heart or the cardiac substructures, number of pack years smoked, diabetes, COPD, hypercholesterolemia and hypertension was not associated with cardiac events after radiotherapy. Figure 3 illustrates that radiation dose distribution is similar between patients who experienced cardiac events and those who did not (P=0.68).

Discussion
In this study, we investigated the impact of radiation dose to cardiac substructures and the whole heart and the development of cardiac events in early-stage lung cancer treated with SBRT. There is evidence that radiation dose to certain structures of the heart contributes to the development of cardiac toxicity (6,15,24). However, our analysis did not find statistically significant correlations between radiation dose to the total heart or its substructures and overall survival or development of cardiac events. We observed that doses to the heart and its substructures show large variability among patients. As expected, there was an inverse correlation of radiation dose with the distance of the tumor from the heart, although this correlation was weak. Radiotherapy to centrally-located or large tumors did not necessarily result in higher cardiac dose compared to peripherally-located and smaller tumors. Furthermore, more advanced radiation treatment technology did not necessarily spare the heart from excessive radiation dose. While in our study patients with preexisting cardiac conditions were more likely to develop cardiac events, patients with other medical comorbidities were not. These findings will add to the understanding of cardiac events following SBRT in early-stage lung cancer.
Radiotherapy-induced cardiac toxicity
In breast, lymphoma and locally-advanced lung cancer, standard fractionation is typically utilized as opposed to SBRT which is studied here (2-4,7). High doses with SBRT only involve a small volume of the heart. For tumors located close to the heart, the dose to a small area can be fairly high. Because of this, exploring the dose to individual cardiac substructures is important (6,9). Indeed, it is currently unknown how much radiation dose is safe to administer to the heart under these circumstances (25). Higher radiation doses to the left ventricle (6), LAD (25) and left atrium (15) have been associated with an increased rate of post-radiotherapy cardiac events which is mainly postulated to be due to ischemic changes. Pericardial disease, conduction system disorders and cardiomyopathy have been identified as radiotherapy-induced cardiotoxicities as well, though to a lesser extent (8). If a substructure that is particularly sensitive to radiation therapy is identified, SBRT technique can be used to create a dose distribution that avoids specific substructures as much as possible (11,25).
Cardiac toxicity following SBRT has rarely been reported. Only two other studies investigated radiation dose to the heart in early stage lung cancer treated with SBRT. The follow-up times in those studies were comparable to our report (18,19). Tembhekar et al. show results similar to ours. Cardiac dose was not associated with overall survival in lung cancer SBRT (18). The dose-volume parameters of the heart in this study are comparable to ours (MHD 1.57 Gy compared to 2.26 Gy in ours and maximum heart dose 14.07 Gy compared to 15.64 Gy in ours). The second much larger study including 803 patients by Stam et al. showed that maximum dose to the left atrium and the dose to 90% of the superior vena cava were significantly associated with non-cancer death (19). The pathophysiologic mechanism behind this observation is not clear (26). The dose-volume parameters of the heart in this report are considerably different from ours [MHD was 11.40 Gy (EQD2) compared to 1.90 Gy (EQD2) in ours and left atrium maximum dose was 6.50 Gy (EQD2) compared to 3.38 Gy (EQD2) in ours], while tumor sizes were similar. Stam et al. did not investigate any association with cardiac events. The causative relation of high doses with cardiac events and cardiac mortality is therefore still unclear. However, the present study provides more detailed analysis on cardiac dose and patient outcomes after SBRT in early-stage lung cancer. Interestingly, in our cohort no pericardial effusions were observed, which is commonly observed after radiotherapy for locally advanced lung cancer (14).
Preexisting cardiac conditions
Five-year overall survival for early-stage lung cancer patients treated with SBRT is only 44% which underscores the relevance of non-cancer contributors to mortality (17). At 30 days, cardiovascular disease has been reported as the most frequent cause of death in early-stage NSCLC patients (23). In our study, patients with preexisting cardiac conditions were more likely to develop cardiac events after radiation therapy. Notably, in our patient population various comorbidities were more common than in most patients who are treated for early-stage lung cancer. Therefore most of the patients in our cohort were medical inoperable. While the association between cardiac event-free survival and preexisting heart conditions did not reach statistical significance—likely due to insufficient patient numbers—there was a clear separation of the Kaplan-Meier curves (Figure 2B). Thus, the relation between cardiac dose and radiotherapy-related heart toxicity remains unclear and further clinical studies are needed to determine how much care should be taken to avoid delivering potentially detrimental radiotherapy doses to the heart and its substructures. This includes assessment of interfraction variations of dose delivery to the heart (24). Our findings indicate that patients with preexisting heart conditions need to be observed with great care as they are at a higher risk for cardiac events which might or might not be related to SBRT.
Other medical comorbidities
There is evidence that medical comorbidities, particularly COPD, play a role in lung cancer mortality (23,27). However, the role of comorbidities in early-stage lung cancer treated with radiation therapy is a relatively unexplored area. In our cohort, we did not identify any association between diabetes, high cholesterol, COPD, hypertension, smoking status and the development of cardiac events. In addition, longer follow-up times might be needed to fully characterize cardiac events after SBRT. For locally advanced lung cancer, post-radiation therapy cardiac events occurred at a median of 26 months after therapy, but happened as early as one month (7), whereas in our study cardiac events were observed after on average 19 months (3–60 months). Therefore, even with rather short follow-up times of 35 months on average, early cardiac events can be identified.
Limitations
Similar to the other two studies on SBRT and cardiac toxicity (18,19), ours is also limited by its retrospective nature. Further investigations are needed to determine whether radiation dose to the heart or individual substructures lead to the development of cardiotoxicity and mortality in early-stage lung cancer. Longer follow-up, larger patient samples, prospective study design and contouring of individual cardiac substructures are essential. Echocardiography, coronary computed tomography and angiography may be helpful to predict which patients are likely to develop cardiac events early after treatment (26). Radiation-induced cardiac disease was reported to be associated with marked thickening, fibrosis and narrowing of coronary arteries (28) and by radiation-induced perfusion defects (29). However, these studies were done using standard fractionation radiotherapy and not SBRT. Learning more about the mechanism of radiation-induced heart damage may lead to the development of cardioprotective medications, such as possibly ROS scavengers, and to the discovery of biomarkers for cardiotoxicity (26,30).
Conclusions
Doses to the heart and its substructures show large variability and depend on the tumor location in early-stage lung cancer. New cardiac events after SBRT were associated with patients’ history of heart problems. Lung cancer patients with preexisting heart conditions need to be watched closely as heart disease might affect morbidity and potentially influence survival. Longer follow-up times and greater patient samples may be needed to identify correlations between comorbidities, radiation technique or cardiac radiation doses with cardiac events or overall survival.
Acknowledgements
We thank Dr. Nitai Mukhopadhyay for biostatistical support.
Footnote
Conflicts of Interest: GD. Hugo: research funding Philips, NCI, license agreement Varian. E Weiss: research funding Philips and Varian Medical Systems, NCI, royalties from UpToDate. The other authors have no conflicts of interest to declare.
Ethical Statement: Approval for this study was obtained from Virginia Commonwealth University Internal Review Board (VCU IRB HM15356). Patient consent was waived.
References
- Darby SC, Ewertz M, McGale P, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557-65. [Crossref] [PubMed]
- Bouillon K, Haddy N, Delaloge S, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol 2011;57:445-52. [Crossref] [PubMed]
- Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s Disease. Cancer 1976;37:2813-25. [Crossref] [PubMed]
- Tukenova M, Guibout C, Oberlin O, et al. Role of Cancer Treatment in Long-Term Overall and Cardiovascular Mortality After Childhood Cancer. J Clin Oncol 2010;28:1308-15. [Crossref] [PubMed]
- van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol 2017;35:1171-8. [Crossref] [PubMed]
- Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387-94. [Crossref] [PubMed]
- Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract 2011;2011:317659. [PubMed]
- Wollschläger D, Karle H, Stockinger M, et al. Radiation dose distribution in functional heart regions from tangential breast cancer radiotherapy. Radiother Oncol 2016;119:65-70. [Crossref] [PubMed]
- Simone CB. New Era in Radiation Oncology for Lung Cancer: Recognizing the Importance of Cardiac Irradiation. J Clin Oncol 2017;35:1381-3. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non–Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol 2017;12:293-301. [Crossref] [PubMed]
- Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395-402. [Crossref] [PubMed]
- Ning MS, Tang L, Gomez DR, et al. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:70-9. [Crossref] [PubMed]
- Vivekanandan S, Landau DB, Counsell N, et al. The Impact of Cardiac Radiation Dosimetry on Survival After Radiation Therapy for Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:51-60. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. Ca Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Tembhekar AR, Wright CL, Daly ME. Cardiac dose and survival after stereotactic body radiotherapy for early-stage non-small-cell lung cancer. Clin Lung Cancer 2017;18:293-8. [Crossref] [PubMed]
- Stam B, Peulen H, Guckenberger M, et al. Dose to heart substructures is associated with non-cancer death after SBRT in stage I-II NSCLC patients. Radiother Oncol 2017;123:370-5. [Crossref] [PubMed]
- Feng M, Moran JM, Keolling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10-8. [Crossref] [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [Crossref] [PubMed]
- Jones B, Dale RG, Deehan C, et al. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13:71-81. [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Jan N, Guy C, Reshko LB, et al. Lung and Heart Dose Variability During Radiation Therapy of Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;98:683-90. [Crossref] [PubMed]
- Nieder C, Schill S, Kneschaurek P, et al. Influence of different treatment techniques on radiation dose to the LAD coronary artery. Radiat Oncol 2007;2:20. [Crossref] [PubMed]
- Bhattacharya S. Asaitthamby. Ionizing radiation and heart risks. Semin Cell Dev Biol 2016;58:14-25. [Crossref] [PubMed]
- Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015;3:631-9. [Crossref] [PubMed]
- Brosius FC 3rd, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med 1981;70:519-30. [Crossref] [PubMed]
- Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005;63:214-23. [Crossref] [PubMed]
- D'Errico MP, Grimaldi L, Petruzzelli MF, et al. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e239-46. [Crossref] [PubMed]


