Factors associated with the prognosis and long-term survival of patients with metastatic lung adenocarcinoma: a retrospective analysis
Introduction
Lung cancer is the most common and deadliest cancer worldwide (1). During the era of platinum-based combination chemotherapy patients diagnosed with advanced non-small cell lung cancer (NSCLC) experienced poor outcomes, with a typical expected lifetime of around 8 to 13 additional months (2-8). Driver mutations in the epidermal growth factor receptor (EGFR) gene have been found in a subset of lung adenocarcinomas and define cancers in which tumor cell survival is exquisitely dependent on EGFR pathway signaling (9). Furthermore, patients with advanced NSCLC harboring oncogenic driver mutation had better survival outcome as comparing to patients without driver mutation (10,11). In Asia-Pacific region, EGFR exon 18–21 mutation is the most common type of oncogenic mutation in lung adenocarcinoma, with incidence rate of 55% (12). Tyrosine kinase inhibitors (TKIs) targeting EGFR had been shown to improve progression-free survival in patients with advanced NSCLC harboring sensitive EGFR mutation status in first line setting (13-20). The overall survival had improved to 21–28 months in the era of EGFR TKI.
Despite the overall poor prognosis, a small subgroup of patients with advanced NSCLC would survive more than 5 years from the literature (21-23). Treatment with EGFR TKI is reported to be significant long-term survival factor in one study (23). In rare cases, survival for more than 10 years has been reported in patients with advanced NSCLC who had been treated with chemotherapy or EGFR TKI (24). As the development of TKI in NSCLC, the true impact of EGFR gene and TKI in long term survival remains uncertain. This study aimed to explore the prognostic factors of long-term survival in patients with advanced NSCLC in the era of EGFR TKI.
Methods
Patient selection
There were 1,081 patients with stage IV lung adenocarcinoma included for this study. All the diagnosis was made with pathologic proof between 2005–2009 at our institute. We excluded patients with tumor histology type other than adenocarcinoma (N=1,692), received subsequent treatment at other hospital (N=37), incompatible clinical stage (N=9), malignancy other than primary lung cancer (N=5) were excluded. A total 1,030 patients were included for final analysis. All the diagnoses were made based on either cytology or histopathology. Long-term survivors (LTS) were defined as patients who survived five years or more after the initial diagnosis of stage IV disease. Survival time was defined from the diagnosis of advanced disease to death of any cause or last follow-up date. Patients who did not survive more than five years were defined as non-LTS group. All patients were diagnosed with stage IV cancer as defined by the American Joint Committee on Cancer (AJCC) 7th edition of cancer staging (25). All clinical information and histopathologic features were collected from electric medical records retrospectively. The study was approved by the institutional review board (approval number 201800208B0), in compliance with the Helsinki Declaration (1996).
Data collection
Data on patient age, gender, smoking status, performance status at diagnosis, TNM stage, metastatic pattern, number of metastases, and organs with tumor involvement, EGFR exon 18–21 gene mutation status, types of treatment received, EGFR TKI treatment and longest EGFR TKI duration were retrospectively obtained from medical charts. Extrathoracic involvement was defined as having metastasis to any of the following organ sites at diagnosis: adrenal gland, bone, central nervous system, cutaneous soft tissue, liver, non-regional lymph nodes, peritoneum, skin and spleen.
Statistical analysis
Basic demographic data were summarized as the n (%) for categorical variables and medians with ranges or 95% confidence interval (CI) for continuous variables. We calculated the odds ratio (OR) of long-term survival from collected clinical parameters to predict long-term survival. A binary logistic regression model was used for multivariate analysis. All statistical assessments were two-sided. Survival time was analyzed using the Kaplan-Meier method. Log-rank tests were used to determine significant differences between the survival curves. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered significant.
Results
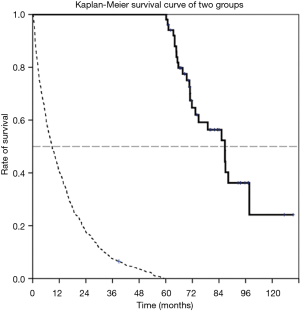
A total 52 LTS and 978 non-LTS were identified during a five-year retrieval period (Jan 2005 to Dec 2009). The median survival time was 86.6 months for the LTS patient group (95% CI: 75.9–97.3 months) and 7.0 months in the non-LTS group (95% CI: 6.16–7.85). The basic characteristics of the two groups are listed in Table 1. Patients in the LTS group had a significantly better performance status when comparing with the non-LTS group [Eastern Cooperative Oncology Group (ECOG); 0–1 96.0% vs. 67.9%, P<0.001]. Smoking status was not significantly different between the two groups (82.7% vs. 69.9%, P=0.126). The outcome and the Kaplan-Meier survival curve of two groups are shown in Figure 1.
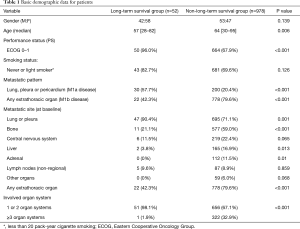
Full table
Regarding the metastatic pattern, patients had more contralateral or pleural/pericardial metastases in the LTS group than in the non-LTS group (51.9% vs. 19.0%, P<0.001), while less extrathoracic spread than in the non-LTS group (42.3% vs. 79.6%, P<0.001). The metastatic patterns are also shown in Table 1. The number of involved organs was significantly lesser in the LTS group than in the non-LTS group (three or more organs 1.9% vs. 32.9%, P<0.001). The metastatic sites at baseline shown in Table 1 also had significant differences in the two groups. Commonly involved organ sites in the LTS group included the lung and pleura (90.4% vs. 71.1%, P=0.001), bone (21.1% vs. 59.0%, P<0.001), liver (3.8% vs. 16.9%, P=0.013), and adrenal gland (0% vs. 11.5%, P=0.010) when comparing with the non-LTS group.
The EGFR mutation of tumor and treatment type is summarized in Table 2. The mutation of the EGFR gene was identified more frequently in the LTS group than in the non-LTS group (19.2% vs. 7.1%, P=0.006). Specific EGFR mutations included in-frame deletion in exon 19, L858R in exon 21, L861Q in exon 21, and insertion in exon 19. Patients in the LTS group received significantly more treatments than the non-LTS group, including metastasectomy (30.8% vs. 11.6%, P<0.001), any chemotherapy (88.5% vs. 64.0%, P<0.001) and EGFR TKI therapy (94.2% vs. 54.9%, P<0.001).
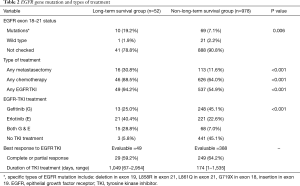
Full table
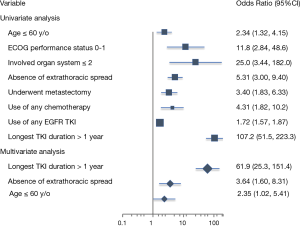
The ORs associated with long-term survival are listed in Table 3. Significant factors associated with long-term survival identified in our series were age younger than 60 years (OR 2.34, 95% CI: 1.32–4.15, P=0.003), performance status ECOG 0–1 (OR 11.75, 95% CI: 2.84–48.59, P<0.001), absence of extrathoracic spread (OR 5.31, 95% CI: 3.00–9.40, P<0.001), underwent metastasectomy (OR 3.40, 95% CI: 1.83–6.33, P<0.001), use of any chemotherapy (OR 4.31, 95% CI: 1.82–10.20, P<0.001), use of EGFR TKI (OR 1.72, 95% CI: 1.57–1.87, P<0.001), and longest EGFR TKI duration more than 1 year (OR 107.2, 95% CI: 51.46–223.3, P<0.001). In multivariate analysis using binary logistical regression model, significant factors associated with long-term survival were age younger than 60 years (adjusted OR 2.35, 95% CI: 1.02–5.41, P=0.044), absence of extrathoracic spread (adjusted OR 3.64, 95% CI: 1.60–8.31, P=0.002) and longest EGFR TKI duration more than 1 year (adjusted OR 61.9, 95% CI: 25.32–151.4, P<0.001). The forest plot of OR was shown in Figure 2.
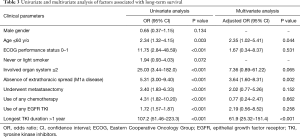
Full table
Discussion
To our knowledge, this is the largest retrospective study to report long-term survival (more than five years) of patients with advanced NSCLC in the era of EGFR TKI. In this study, we reported a five-year survival of 5.0% (52/1,030) in patients diagnosed with metastatic lung adenocarcinoma. Wang et al. (22) reported a five-year survival of 6.6% in 846 patients with stage IIIA-IV NSCLC in the era of chemotherapy. Ozkaya et al. (21) and Kaira et al. (23) also reported five-year survival rates of 2.8% (total number =141) and 8.0% (total number =124), respectively, in patients with stage IIIB-IV NSCLC.
In recent studies focusing on long-term survival of advanced NSCLC, treatment of EGFR TKI was reported as a significant factor to LTS in several retrospective studies (23,26). In this study, we identified three significant prognostic factors associated with LTS, which were age 60 years old or younger, absence of extrathoracic spread (M1a disease) and longest TKI duration more than one year. Among patients with all these three clinical factors in this study, the five-year survival rate was 100% (14 of 14). These findings suggested that disease pattern and the response to treatment play a major role of having long-term survival. Besides systemic therapy, patients with oligometastases disease might be amenable to a curative surgery or localized therapy (27), which also played a role in achieving long-term survival. Other factors associated with long-term survival (more than five years) from other retrospective studies are summarized in Table 4.

Full table
Among phase III trials comparing single-agent EGFR TKI to standard platinum-based doublet chemotherapy in treating patients with advanced NSCLC as first-line setting, the median overall survival was reported between 19.3 to 30.5 months (13-20,28). However, a subgroup of patients had achieved longer clinical benefit from EGFR TKI. In a recently reported study, the medicine Afatinib has been reported to provide a significantly higher 24-month progression-free survival rate than the medicine Gefitinib in first-line setting (17.6% vs. 7.6%) (29), indicating that a different TKI might have an effect on the clinical outcome. However, only a small part of patients in this study had tests of tumor EGFR gene mutation, with an overall majority (90.2%) having had unknown EGFR mutation status. This is the major limitation of our study. It remains uncertain whether patients with metastatic NSCLC acquiring EGFR mutation would had a higher long-term survival rate. However, patients with evaluable disease in LTS group treated with EGFR TKI had objective response rate of 59% (29/49). In a phase III clinical trial study comparing gefitinib to carboplatin plus paclitaxel (20), the objective response rate of gefitinib was 71.2% in EGFR gene mutation-positive subgroup (P<0.001) and 1.1% in EGFR gene mutation-negative subgroup (P=0.001). Such distinct response to gefitinib suggested that LTS group in our study is very likely a group of EGFR-mutant lung adenocarcinoma.
Anaplastic lymphoma kinase (ALK) fusion oncogene is an emerging molecular target in lung adenocarcinoma (30), which accounts for 3–5% of genetic alteration (12,31,32). Patients with ALK fusion oncogene were characterized by younger age, non-smoking status and good response to several TKIs (33). Surprisingly, a retrospective study includes 14 patients with advanced NSCLC harboring ALK fusion gene reported five-year OS rate of 78.6% (34). ROS1 rearrangement is another emergent but rare molecular target (35) that respond well to crizotinib in advanced NSCLC (36). KRAS mutation is the major genetic alteration in lung adenocarcinoma of Western countries (37). In contrast, KRAS mutation appears to negatively affect overall survival (38,39). However, our study is also limited to identify the potential long-term survival impact of ALK, ROS1, KRAS and other oncogenic mutations.
This retrospective study has several limitations. First, only patients with adenocarcinoma histology were included in this study. Therefore, the interpretation of the results may be inappropriate to patients with squamous cell carcinoma or other histology types. Second, the overall survival outcome of non-LTS group in this study is lower than described in literature. This probably relates to poor performance status (32.1% patients with performance status ECOG ≥2), who is not fit for standard chemotherapy. Third, EGFR TKI was not reimbursed as second-line setting (regardless of EGFR mutation status) in Taiwan before 2008. Therefore, not all patients had received EGFR TKI in this study, which precludes the efficacy and response to EGFR TKI from certain patients and introduced a selection bias in this study.
Conclusions
In conclusion, our results suggest that an age younger than 60 years, absence of extrathoracic spread and EGFR TKI treatment duration of more than one year play an important role in the long-term for survivors who survive for more than 5 years.
Acknowledgements
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help. The authors also thank professor Alex Yuang-Chi Chang for his great assistance and help.
Funding: The study and data collection processes were funded by the grants from the CGMH foundation (Grant No. CORPG3F0721) to John WC Chang from Chang Gung Memorial Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed and approved by our Institutional Review Board (approval number 201800208B0), in compliance with the Helsinki Declaration (1996).
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Perol M, Chouaid C, Perol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012;30:3516-24. [Crossref] [PubMed]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24. [Crossref] [PubMed]
- Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2000;18:122-30. [Crossref] [PubMed]
- Gatzemeier U, von Pawel J, Gottfried M, et al. Phase III comparative study of high-dose cisplatin versus a combination of paclitaxel and cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 2000;18:3390-9. [Crossref] [PubMed]
- Ardizzoni A, Antonelli G, Grossi F, et al. The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC). Ann Oncol 1999;10 Suppl 5:S13-7. [Crossref] [PubMed]
- Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 1998;16:2459-65. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Hsu KH, Ho CC, Hsia TC, et al. Identification of five driver gene mutations in patients with treatment-naive lung adenocarcinoma in Taiwan. PLoS One 2015;10:e0120852. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Ozkaya S, Findik S, Dirican A, et al. Long-term survival rates of patients with stage IIIB and IV non-small cell lung cancer treated with cisplatin plus vinorelbine or gemcitabine. Exp Ther Med 2012;4:1035-8. [Crossref] [PubMed]
- Wang T, Nelson RA, Bogardus A, et al. Five-year lung cancer survival: which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer 2010;116:1518-25. [Crossref] [PubMed]
- Kaira K, Takahashi T, Murakami H, et al. Long-term survivors of more than 5 years in advanced non-small cell lung cancer. Lung Cancer 2010;67:120-3. [Crossref] [PubMed]
- Kempf E, Planchard D, Le Chevalier T, et al. 10-year long-term survival of a metastatic EGFR-mutated nonsmall cell lung cancer patient. The European respiratory journal 2015;46:280-2. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Satoh H, Ishikawa H, Ohara G, et al. Long-term survivors after chemotherapy in advanced non-small cell lung cancer. Anticancer Res 2007;27:4457-60. [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Huang CY, Chen TC, Huang SW, et al. Epstein–Barr virus (EBV) associated lymphoepithelioma-like cholangiocarcinoma with elevated EBV DNA titer and treated with systemic chemotherapy. Journal of Cancer Research and Practice 2017;4:19-22. [Crossref]
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [Crossref] [PubMed]
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Murakami H, Ono A, Nakashima K, et al. Long-term clinical outcomes of ALK inhibitors in patients with ALK-positive advanced non-small cell lung cancer. J Clin Oncol 2017;35:abstr e20542.
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Metro G, Chiari R, Bennati C, et al. Clinical outcome with platinum-based chemotherapy in patients with advanced nonsquamous EGFR wild-type non-small-cell lung cancer segregated according to KRAS mutation status. Clin Lung Cancer 2014;15:86-92. [Crossref] [PubMed]
- Chen YF, Hsieh MS, Wu SG, et al. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol 2014;9:1171-9. [Crossref] [PubMed]


