An acoustic method for systematic ventricular assist device thrombus evaluation with a novel artificial thrombus model
Introduction
Mechanical circulatory support with left ventricular assist devices (LVADs) is a state of the art therapy for the treatment of end stage heart failure, either as bridge-to-transplantation or as a destination therapy. Pump technology development over the years from pulsatile pumps to continuous flow devices has led to improved outcomes. Nevertheless infection, stroke and pump thrombosis (PT) remain important limitations in long-term LVAD support (1,2). Improved clinical management (3-5), innovative surgical techniques (6-8) as well as third generation pump designs including contactless suspension of the rotor (9-11) were important improvements but could not eliminate PT. The introduction of foreign material to the human body in combination with non-physiologic flow conditions can lead to activation of coagulation, and subsequently increase the risk of thrombus formation depending on patient-specific and management-specific factors (12,13). Thrombus deposits, consisting mainly of fibrin and platelets, can be found inside the device (inflow cannula, outflow graft or pump cavity) and contribute to a loss of performance. VAD thrombosis can result in significant hemolysis, stroke, decreased pump performance and hence insufficient ventricular support. Since medical therapy such as thrombolysis is often ineffective, advanced PT in patients routinely calls for risky and expensive pump replacement surgery. During replacement surgery an exchange from one to another LVAD type is feasible (14,15), in order to ensure that the patient is supported with the newest pump technology. Nevertheless, pump exchange carries the inherent risk of highly invasive open heart surgery and does not guarantee freedom of PT. Early diagnosis of PT is therefore critical to allow for timely non-invasive therapeutic interventions such as thrombolysis. Currently PT is often indirectly detected by elevated VAD power consumption and pathological laboratory parameters when thrombus consolidates in the pump (16-18). Innovative diagnostic methods such as acoustic spectrum-based thrombus detection are now being introduced into clinical care with promising preliminary results (19,20). However, in vitro studies are required to verify and validate their specificity and sensitivity under standardized conditions. Such studies require robust, reproducible and easily available thrombus models, which accurately simulate acoustic properties of physiological thrombus conditions.
In the present study, an artificial thrombus model was developed and established for the validation of acoustic spectrum-based diagnosis of pump thrombus in an LVAD system. An LVAD was integrated into a simulated blood-flow reactor under controlled physiological parameters such as fluid pressure, flow and viscosity. Various materials were employed and their suitability as a thrombus replacement material was subsequently experimentally determined. The most suitable thrombus replacement material was used to mimic physiological PT in this acoustic study.
Methods
Artificial thrombus model
For the artificial thrombus model (aTM), silicone (Bad Silikon wie Gummi, LUGATO) was chosen. This material meets the specifications to mimic a human thrombus build in the pump, which are density comparable to a pathologic thrombus (1,080 g/L, comparable to human blood with a theoretical hematocrit of 100%) (21), resistance to shear stress with magnitude of 1,500 Pa (which is the maximum shear stress of 500 Pa expected in a running state of a rotary blood pump multiplied by a safety factor of three) (22). Also, the silicone material meets further requirements with regard to the applicability in the in vitro test setting for pump thrombus acoustic and vibrational studies: Consistency in measurement readings (pump parameters and frequency peak numbers from acoustic analysis) over 10 minutes; ease of application to the rotor in minimal increments of 0.5 mg with a maximum variation of 0.1 mg and residue-free removal after a series of measurements; hazard-free handling and efficient waste disposal.
Figure 1 shows a rotor with a typical thrombus explanted from a patient and a rotor prepared with aTM for the study (5 mg).

Mock loop setup
For pump thrombus acoustic and vibrational studies, a mock circulation loop was used to simulate patient circulation (Figure 2). Two cylinders were connected with flexible tubing. One of them served as a fluid reservoir and to set the preload, the other one served as a compliance chamber. The fluid reservoir was open to atmospheric pressure, while the compliance chamber was closed. To achieve a pulsatile flow, a programmable peristaltic pump from a heart lung machine was used. Two pressure sensors and an ultrasonic flow sensor monitored these settings. A 39% (v/v) glycerin solution served as blood substitute (23 °C, viscosity of 3.3 mPas). A total volume of 2.5 liters was prepared. The LVAD type used in this study was HVAD (“HeartWare”, Medtronic, Inc.). Adjusted pump operation conditions were based on previous literature as follows: LVAD speed (2,700 rpm), mean afterload (96±5 mmHg), mean pressure head (80±5 mmHg), and mean systemic flow (5 L/min) (23).
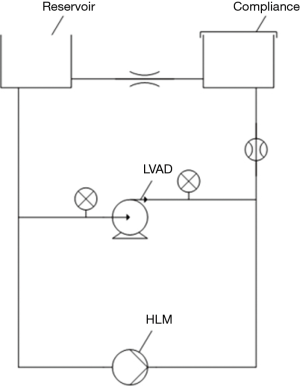
Acoustic measurements
The focus of this study was to investigate the acoustic effects of thrombus inside a LVAD. As described above, silicone was determined to be a suitable aTM material. A measurement system designed by our group was used for acoustic recordings. The system includes a microphone (Sennheiser GmbH, related software: Brüel and Kjäer Vibro) connected to a custom made stethoscope chest-piece. The acoustic measurements were carried out in the mock loop described above. Four explanted, decontaminated, and cleaned LVADs (HVAD, HeartWare, Inc.) were used in this study. To record the frequency spectra the stethoscope was placed at a defined distance from the LVAD.
Prior to each measurement, background noise in the laboratory was recorded for later subtraction. The microphone was calibrated at a frequency of 1,000 Hz. Each measurement lasted for 30 seconds and recorded a frequency spectrum between 0 and 3,200 Hz with 3,200 measuring points. Data were linearly averaged and set against Hanning filter after 100 arithmetic operations. Measurements were carried out with a sample rate of 8,192 Hz. Furthermore, a waiting period of 30 seconds was observed to allow the HVAD to reach constant readings after adjusting pump parameters like flow and pressure.
As a reference, baseline acoustic profiles of the HVADs without thrombus were recorded. Twenty measurements were recorded for each HVAD to determine a mean amplitude characteristic dependent on frequency as well as the corresponding standard deviation.
The aTM was applied in different masses (0.5, 1, 2, 3, 4, 5 mg) to the rotor on the tilted pad (ToR), underneath the rotor in the secondary flow path (TuR) and in the primary flow path in between the blades (TiC) for acoustic impact testing. In the ToR as well as the TuR position, the aTM was applied to the tilted pad and on a comparable position on the opposite surface and carefully spread in rotational direction, respectively. A central placement within the channel was performed in TiC position. The placements are further illustrated in Figure 3.
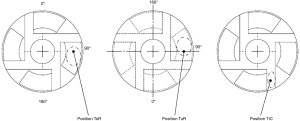
The aTM was applied using a small plastic rod while the rotor (disassembled from pump housing) was positioned on top of an inverted beaker on a set of micro scales. The beaker prevented influence of the strong magnetic field of the HVAD on the readings of the digital scale.
During the test, readings were taken at 30, 105 and 180 seconds. Each thrombus position was tested three times for each LVAD used.
The frequency spectrum of the measurements was examined. A peak was defined as an amplitude excursion with a difference of at least +3 dBb in comparison to both neighboring amplitudes.
The resulting thrombus profiles were compared to the baseline profile of the HVAD and assessed for statistical significance (significance level P=0.05). The number of peaks within the frequency spectrum was observed. Particular attention was paid to the third harmonic (135 Hz according to 2,700 RPM) which suggested a PT in algorithms described in previous studies (20). The spectra were analyzed regarding noticeable patterns and similarities across the HVADs. Furthermore, pump flow and speed were recorded. Data were analyzed with MS Excel 2007 and GraphPad PRISM.
Results
Baseline measurement
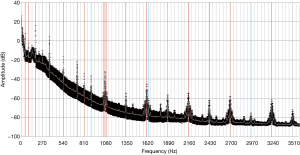
Baseline measurements were performed to identify pump-independent patterns of a thrombus-free pump. Recorded power consumption and estimated flow were highly consistent amongst the measurements with each pump. In addition to LVAD parameters the dominant frequencies (“frequency peaks”, FP) were determined in the frequency spectra. The four pumps presented 28±4 FPs up to 35±6 FPs (4 pumps, 20 baseline measurement repetitions with each pump). Frequency spectra of all were analyzed for similarities. In 80% of the analyzed frequency spectra, 16 specific dominant frequencies could be determined out of which 13 were harmonics of the fundamental frequency (45 Hz according to 2,700 RPM). In 75% of the analyzed frequency spectra, 4 more dominant frequencies could be determined. In 62.5% of the analyzed spectra, the third harmonic could be detected. Figure 4 shows the mean frequency spectrum of one pump with standard deviation of the baseline measurement (n=20).
aTM measurements
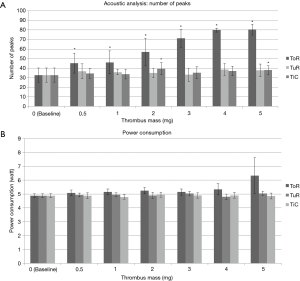
Four pumps were tested with an artificial thrombus, with masses ranging from 0.5 to 5 mg. Three independent experiments were carried out with each pump/thrombus mass combination (n=12). Results for acoustic and power consumption measurement are summarized in Figure 5.
Thrombus in secondary flow path
The power consumption mean values were fairly stable from the baseline measurement (4.9±0.14 W) up to the 5 mg thrombus measurement (5.0±0.15 W). Acoustic measurements with analysis of number of peaks resulted in slightly increased number of peaks compared to baseline (32.7±7.4) varying between 33.1±6.8 within 3 mg thrombus measurements and 38.5±6.3 in 4 mg thrombus measurements, however not significantly different from baseline measurements (P>0.05).
Thrombus in the rotor channel (primary flow path)
The power consumption mean values were fairly stable from baseline measurement (4.9±0.14 W) up to 5 mg thrombus measurement (4.9±0.21 W). Acoustic measurements with analysis of number of peaks revealed a slight increase in the number of peaks compared to baseline (32.7±7.4). Only 2 mg (39.4±6.4) and 5 mg (37.9±4.5) thrombi resulted in significantly increased measurements compared to baseline (P<0.05).
Thrombus on tilted pad
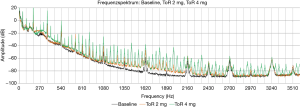
The power consumption mean values were relatively stable from the baseline (4.9±0.14 W) up to 3 mg thrombus (5.1±0.23 W). While 4 mg thrombus resulted in slightly increased power consumption (5.3±0.4 W), 5 mg thrombus resulted in a highly increased power consumption with high standard deviation (6.3±1.29 W); P<0.01. Acoustic measurements with analysis of number of peaks resulted in significant (all P<0.01) and steady increase in the number of peaks compared to baseline (32.7±7.4) from 0.5 mg (45.3±10.4) up to 5 mg thrombus (80.4±5.5). Figure 6 shows representative frequency spectra from measurements of one pump without thrombus (baseline) and with artificial thrombi (2 and 4 mg). The increased number in frequency peaks in data with 2 and 4 mg aTM respectively can be visually assessed.
Discussion
The results of this study in part corroborate and expand previous data (20). While analyzing the recorded baseline data characteristic peaks could be identified. The second (90 Hz), fourth (180 Hz) and superior harmonics were also determined. Kaufmann et al. proposed a thrombus detection algorithm using the existence of the third harmonic as an indicator for thrombus in HVADs, which was confirmed after explantation of the pump. Importantly, this could not be confirmed by our study. Within our data set, the third harmonic was present in 62.5% (50 of 80) of all baseline measurements without any thrombus. This specific frequency was increasingly detected with an aTM. In 180 out of 216 (83.3%) measurements with an aTM a third harmonic was measured. Hence we propose another analysis algorithm using the overall number of peaks as a measure of thrombus existence and weight.
Elevated power consumption is reported as one of the first signs for VAD thrombosis and is part of current diagnostic algorithms (16-18). The HVAD ‘Instructions for Use’ recommends a high watt alarm level of 2 W above mean power consumption, which was adopted by the thrombus detection algorithm from Goldstein et al. (18). Kaufmann et al. recommend alarm levels of 1 W above mean power consumption for earlier pump thrombus detection (20). Our data suggest that within these levels a massive thrombus of 5 mg and bigger can already be present. This corroborates the findings of Kaufmann et al. A thrombus of this size might already be more resistant to thrombolytic therapy, which demonstrates the need for more sensitive algorithms. The acoustic algorithm suggested in this paper was able to detect thrombus of a weight of already 0.5 mg. Röbesaat et al. developed algorithms using the power consumption data in in silico studies (24). These algorithms may also detect an increase in power consumption and therefore thrombus formation earlier compared to the high watt alarms, but thrombus weight or sizes were not investigated.
In the present study, artificial thrombi were applied to three different locations: on top of the tilted pad (the hydrodynamic bearing), in the secondary flow channel (below the rotor) and between the rotor blades (within the flow channel). The acoustic algorithm using the number of peaks was most sensitive to thrombi located on the tilted pad. Our experience with explanted and investigated HVADs showed thrombi on the tilted pad only or—if this thrombus formation is severe—in combination with a thrombus below the rotor due to mechanical contact of rotor and housing. Another observed thrombus location is the thrombus in the channel between the rotor blades, also in combination with a thrombus in one or both of the other locations. It is possible that these thrombi were not formed inside the channel but washed from the ventricle inside the pump. They are observed to be much bigger and to have a different composition and consistency (more erythrocytes included, sometime tissue involved) than those formed on the tilted pad, see Figure 7. This thrombus type appears to have no effect on the mechanical and vibrational behavior of the pump in a manner that can be detected, if the size is in the tested range (0.5 up to 5 mg).

Conclusions
Physiological and artificial/simulated thrombi affect pump function and blood dynamics, resulting in impaired patient hemodynamic support and hematological damage. Those alterations in pump behavior are detectable by changes in power consumption, vibrations and acoustic emission. We showed that our acoustic analysis algorithm is more sensitive compared to the contemporary increased power consumption-based detection method and was able to detect already ten times lighter thrombi in vitro. This applies to thrombi which are positioned on the tilted pad, observed to be the most prominent location of thrombi in our clinic, when investigated after pump explantation. However, continuative in vitro and in vivo studies are recommended to investigate acoustic emissions in case of thrombi at different locations in one pump and to investigate the transfer of the proposed algorithm to clinical application.
Early detection of thrombi indicates detection of small thrombi, which may not incur clinical manifestations in patients, but may also not yet be strongly consolidated and may accordingly be more susceptible to conservative treatment (25,26). For early detection, we suggest an easy-to-use acoustic system for daily or constant home monitoring of LVAD patients. Such a device should be user-friendly and not bothering the patient, measurement should ideally be automatic, and data transmitted via telemetry (27). For future LVAD generations, an integration of the acoustic/vibrational thrombus measurement system is worth considering. The combination of an early pump thrombus detection device with early treatment may result in improved outcome of LVAD therapy with lower chance of thrombus-related complications and high quality of life for LVAD patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: JD Schmitto and G Dogan are consultants for Medtronic and Abbott. The other authors have no conflicts of interest to declare.
References
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Mahr C, et al. Five-year results of patients supported by HeartMate II: outcomes and adverse events. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [PubMed]
- Maltais S, Kilic A, Nathan S, et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant 2017;36:1-12. [Crossref] [PubMed]
- Netuka I, Litzler PY, Berchtold-Herz M, et al. Outcomes in HeartMate II Patients With No Antiplatelet Therapy: 2-Year Results From the European TRACE Study. Ann Thorac Surg 2017;103:1262-8. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Haverich A. Left Ventricular Assist Devices for Advanced Heart Failure. N Engl J Med 2017;376:1894. [PubMed]
- Maltais S, Anwer LA, Tchantchaleishvili V, et al. Left Lateral Thoracotomy for Centrifugal Continuous-Flow Left Ventricular Assist Device Placement: An Analysis from the Mechanical Circulatory Support Research Network. ASAIO J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Hanke JS, Dogan G, Rojas SV, et al. First experiences with HeartMate 3 follow-up and adverse events. J Thorac Cardiovasc Surg 2017;154:173-8. [Crossref] [PubMed]
- Feldmann C, Chatterjee A, Hanke JS, et al. Novel centrifugal pump for heart failure patients: initial success and future challenges. J Thorac Dis 2017;9:1429-31. [Crossref] [PubMed]
- Netuka I, Sood P, Pya Y, et al. Fully Magnetically Levitated Left Ventricular Assist System for Treating Advanced HF: A Multicenter Study. J Am Coll Cardiol 2015;66:2579-89. [Crossref] [PubMed]
- Schmitto JD, Avsar M, Haverich A. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1463-4. [Crossref] [PubMed]
- Krolitzki B, Mueller M, Glasmacher B.. Validation of a Test Setup for Hemocompatibility Testing of Small Cardiovascular Implants. IJAO 2011;34:705.
- Hanke JS, Rojas SV, Dogan G, et al. First series of left ventricular assist device exchanges to HeartMate 3. Eur J Cardiothorac Surg 2017;51:887-92. [Crossref] [PubMed]
- Gallo M, Trivedi JR, Sobieski MA, et al. Surgical Technique for Ventricular Device Exchange: From HeartMate II to HVAD. ASAIO J 2017;63:364-6. [Crossref] [PubMed]
- Scandroglio AM, Kaufmann F, Pieri M, et al. Diagnosis and Treatment Algorithm for Blood Flow Obstructions in Patients With Left Ventricular Assist Device. J Am Coll Cardiol 2016;67:2758-68. [Crossref] [PubMed]
- Birati EY, Rame JE. Diagnosis and Management of LVAD Thrombosis. Curr Treat Options Cardiovasc Med 2015;17:361. [Crossref] [PubMed]
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [Crossref] [PubMed]
- Deniz E, Roeper G, Reiss N, et al. Digital Stethoskope System-VADoskope: A New Diagnostic Tool to Detect Thrombus Formation. Thorac Cardiovasc Surg 2016.OP242.
- Kaufmann F, Hörmandinger C, Stepanenko A, et al. Acoustic spectral analysis for determining pump thrombosis in rotary blood pumps. ASAIO J 2014;60:502-7. [Crossref] [PubMed]
- Masuzawa T, Ezoe S, Kato T, et al. Magnetically Suspended Centrifugal Blood Pump with an Axially Levitated Motor. Artif Organs 2003;27:631-8. [Crossref] [PubMed]
- Kustosz R, Altyntsev I, Darlak M, et al. The Tin Coatings Utilisation As Blood Contact Surface Modification In Implantable Rotary Left Ventricle Assist Device Religaheart Ro. The Journal of Institute of Metallurgy and Materials Science and Committee on Metallurgy of Polish Academy of Sciences 2015;60.
- Timms DL, Gregory SD, Greatrex NA, et al. A compact mock circulation loop for the in vitro testing of cardiovascular devices. Artif Organs 2011;35:384-91. [Crossref] [PubMed]
- Röbesaat JI, Müller-von Aschwege F, Reiss N, et al. Analysis of LVAD log files for the early detection of pump thrombosis. 2017 IEEE Symposium on Computers and Communications (ISCC), 2017.
- Wert L, Hanke JD, Dogan G, et al. Argatroban administration as therapy for thrombosis in patients with continuous-flow ventricular assist devices. J Thorac Dis 2018;10:S1720-27. [Crossref]
- Schrage B, Grahn H, Wagner FM, et al. Effective treatment with a new protocol using tissue-type plasminogen activator thrombolysis for pump thrombosis with the HVAD device. Eur Heart J Acute Cardiovasc Care 2017.2048872616688418. [Epub ahead of print]. [PubMed]
- Reiss N, Schmidt T, Muller-von Aschwege F, et al. Telemonitoring and Medical Care of Heart Failure Patients Supported by Left Ventricular Assist Devices - The Medolution Project. Stud Health Technol Inform 2017;236:267-74. [PubMed]


