Bioengineered tissue solutions for repair, correction and reconstruction in cardiovascular surgery
Introduction
Cardiovascular diseases are among the leading mortality cause in the World and the use of cardiac patches remains among the main therapeutic solutions for their surgical treatment.
Currently applied cardiovascular patches can be of synthetic origin or biological derivation. Synthetic materials like Dacron® (Koch Industries, Inc., Wichita, KS, USA) are rigid and not flexible, thus unable to contract simultaneously with the beating heart tissue. Moreover, their biocompatibility is poor. In fact, they are known to induce local inflammatory reactions and endocarditis in the recipient patient (1). As a consequence, thickening, fibrotic processes and calcification may occur, as well as no regeneration of the autologous tissue (2).
A greater clinical interest has been devoted to biologically derived patches and substitutes. A large class of biological devices has been applied or is presently under study for the repair, correction and reconstruction of cardiovascular alterations. Autologous and xenogeneic pericardia, porcine intestinal submucosa extracellular matrix (SIS-ECM), as well as tissue engineered myocardium, blood vessels and heart valves have been proposed as more valid alternatives to synthetic materials for several surgical indications.
This review will offer an overview on the different solutions of regenerative medicine tested at the preclinical level and will discuss the outcomes of their clinical use in the cardiovascular surgical field.
Pericardial patches (PP)
Preclinical experiences
The first application of a chemically stabilized animal pericardium dates back to 1971, when the Ionescu-Shiley bioprosthetic heart valve, manufactured with this tissue, was implanted clinically in aortic position (3). The successful results achieved in the following years encouraged the rapid spread of pericardial xenografts in several other cardiovascular applications (4). The wide use in vascular surgery, correction of congenital and acquired heart diseases, and pericardial closure following heart procedures was sustained by its potentially unlimited availability, low antigenicity thanks to glutaraldehyde (GA) shielding, modest infection incidence, easy handling, and minimal suture line bleeding.
The early literature about the chemical modifications and biological behavior of GA was characterized by the paucity of in vitro studies. Initially, cardiac surgeons were more interested about feasibility and functionality of PP implants rather than on their actual biological effects.
Recently, cytotoxicity of commercially available GA-crosslinked bovine pericardial patches (BPPs) was demonstrated by in vitro direct contact and extract assays of murine fibroblast culture. Moreover, the activation of human macrophages associated with the release of inflammatory cytokines was observed (5). These results were documented also for commercial GA-treated xenogeneic heart valves by quantifying the ratio among anti-/pro-inflammatory cytokines (6).
Early in vivo animal experiments were performed mainly in dogs and revealed the most typical drawbacks related to the use of bioprosthetic GA-treated tissues at the early and mid-term evaluation. GA-treated xenografts were tested to close defects of pericardium (7-9) and atria (9-11), as well as substitutes of mitral chordae (8) and aorta (9). These materials progressively caused fibrotic deposition, patch thickening and shrinkage, chronic inflammation, and several grades of calcification not related to the sutures. Adherences to the epicardium were found in case of pericardial substitutes (7,8) and were particularly severe when cardiopulmonary bypass was performed (12). After one year follow up, they appeared, however, attenuated in respect to those generated with synthetic materials (13).
In general, the comparison of different synthetic polytetrafluoroethylene (PTFE), woven Dacron® and biological materials (canine autologous atrium and pericardium, bovine pericardium) revealed that these severe complications were common and independent from the type of substitute (10,11). Furthermore, the distribution of calcific foci was reported as strongly correlated to implantation site, function, and bloodstream contact with these patches (8,9).
Subcutaneous implants in rats allowed to better investigate the correlation between GA exposure and occurrence of calcification, substantiating the association between treatment duration and rate of BPP mineralization (14,15).
Despite the wide use in the last 50 years, there are still open issues and controversial opinions regarding the effective long-term durability and the biocompatibility of aldehyde-treated PPs. Indeed, GA treatment does not completely prevent host’s response and foreign body reactions may occur following implantation (16,17). Nevertheless, the progressive evolution of GA-treated PPs demonstrated their improved performances in terms of hemocompatibility and tissue compliance in respect to the synthetic ones, rendering ordinary their clinical practice.
Clinical applications
Nowadays, the biomedical industry offers a broad range of commercial products based on xenogeneic pericardia, accessible from several species and supplied with different chemical treatments (Table 1). Most of these products are GA-treated in order to induce an immunologic barrier, but also to increase their durability and mechanical stability. Moreover, in the last years, the manufacturers began to finally perceive the critical issues related to the indiscriminate use of chemical crosslinking and responded with anti-calcification treatments and procedures directed to stabilize free aldehydes.
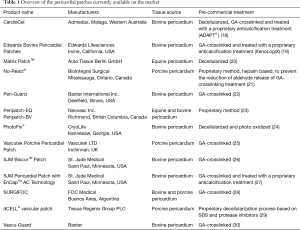
Full table
Although these biomaterials generally present low thrombogenicity and convenient hemodynamics performances, unfavorable outcomes, such as restenosis, intimal hyperplasia, pseudoaneurysmal deformations, shrinkage, rupture, cartilaginous degeneration, thrombosis, dystrophic calcification, and fibrosis, are not rare reported events.
Pericardial closure
One of the early PP applications was the pericardial closure in case of unfeasible primary suture. Although there is a lack of medical consensus on this procedure, it contributes to prevent cardiac herniation, tamponade, and sternum adherences that might increase post- and re-operative mortality and morbidities, such as hemorrhages and damages to the heart and great vessels (31). In the past, several attempts of pericardial closure were performed with synthetic materials, as Silastic (32), Dacron® (33,34), silicone rubber (35), polyurethane (36), and PTFE (36-38), and with biological tissues, as pleura (39), dura mater (34), and fascia lata (40-42). Indeed, PPs of bovine, porcine, and equine origin were mostly used with this aim. As an example, the application of GA-treated porcine PPs (PPPs) was clinically successful after 9-month surgery (7) but, in the long term (more than 4 years), it revealed to cause a severe epicardial reaction (43). Results with GA-treated BPPs were controversial. Yakirevich et al. disclosed freedom from adhesions and immunoreactions in patients re-operated after 7 years (44), while Skinner and colleagues reported a strong epicardial inflammation (45), which rendered necessary the complete patch removal (46). GA-treated equine pericardial patches (EPPs) demonstrated to be a valid alternative, thanks to the low rate of bioprosthesis-related complications and reduced adhesion in recurrent sternotomy (47).
Congenital and acquired heart defects
GA-treated autologous PPs (APPs) or BPPs were used to correct a wide spectrum of congenital heart defects because of their versatility and easy handling. During the surgery of Tetralogy of Fallot, these patches were applied to reconstruct the right ventricular outflow tract (48) but pulmonary incompetence arose due to the absence of contractility (49). The main side effect observed was the aneurysmal dilatation of the bioprosthetic tissue, which was demonstrated to be size-related to the patch itself (50). For equivalent considerations, correction of congenital septal defects using autologous and GA-treated heterologous pericardia might undergo the same post-surgery evolution (51-53). Schoof et al. reported absent inflammation and tissue thickening of chemically untreated APPs, thanks to the progressive adaptation of the biomaterial (54). After 60 months of follow up, no calcifications were disclosed for BPPs used for the correction of various congenital heart defects (55). Conversely, a case report accounted for the early degeneration of a GA-treated BPP used for mitral valve augmentation. Calcification, fibrosis, pannus formation, thickening, neovascularization, disruption of patch microscopic structure but no immunogenic response were described (56). A comparison between GA-fixed BPPs and cryopreserved homografts used for aortic reconstruction in the correction of the hypoplastic left ventricle (LV) demonstrated that xenografts were less immunogenic, had a similar post-operative recurrent obstruction, besides being cost-effective (57).
Other applications of PPs in congenital heart diseases were represented by the corrections of tricuspid insufficiency in Ebstein’s anomaly (58), double outlet of the right ventricle (59,60), complete atrioventricular septum defect (61,62) and reconstruction of the bicuspid aortic valve (63).
PPs were also adopted in valvuloplasty intervention in case of limited amount of autologous tissue. Aortic valvuloplasty with autologous GA-crosslinked APPs generally demonstrated to be effective in the correction of aortic structural defects (fenestration, bicuspid valve) and degenerations (calcification and prolapse) up to 7 years (64). As a result of the GA-treated APP implantation, the timing of re-intervention for pediatric patients was postponed up to 15 years with outcomes superior to the ones obtained in Ross operation and balloon valvuloplasty (65). These biomaterials were also used to enlarge the annulus prior to valve replacement (66).
In the correction of rheumatic disease sequelae, GA-crosslinked APPs served to prevent damages to the conductive system during procedures of tricuspid valve replacement (67), as well as they were adopted to avoid the rupture of the left ventricular myocardium during mitral valve replacement following its calcification (68). Furthermore, in the settings of myocardial infarction, GA-treated APPs (69) or EPPs (70) were applied to elude cardiac rupture.
The dilated wall of ventricular aneurysm might be repaired by isolating the deformation through the endoaneurysmorrhaphy technique. In this case, the application of synthetic substitutes was very often associated with post-operative comorbidities, such as infections and compliance disparity, while the use of crosslinked APPs turned to be more effective, leading to moderate calcifications only (71-73).
As a general consideration, the use of APPs is associated to a lower infection rate, being advantageous also to reconstruct the LV in those patients with severe sepsis (both endocarditis and myocardial abscess) and, hence, considerably reducing the risk of relapses (74). A similar resistance in infected fields was demonstrated for BPPs (75,76). Commonly, the repair of aortic and mitral valves with both bovine and autologous GA-treated PPs increased patient survival over the replacement (77,78).
Vascular surgery
PPs were introduced in vascular surgery in 1991 since their compliance is more similar to native vascular tissues than to artificial grafts. As in other surgical uses, GA treatment helped to stabilize PP tissue and prevent aneurysmal dilatations. In particular, BPPs presented superior hemostatic properties and inferior suture line bleeding in respect to Dacron® (79).
One of the main drawbacks correlated to the use of GA-treated grafts and patches is, however, their challenging endothelialization due to the cytotoxic effects of the gradual and prolonged aldehyde release from the biomaterial surface (80-82). As a consequence, thrombogenicity and graft failure might occur, also as symptomatic of collagen instability with a subsequent predisposition to aneurysmal deformations (83).
In aortic arch and root substitution, GA-treated xenogeneic PPs overcame the limitations of the most common substitute, i.e., Dacron®, such as poor hemostasis and handling. The mid-term follow up evidenced appropriate performances without calcification or tissue deterioration (84). GA-crosslinked PPs were also applied for the reconstruction of valvulated conduits as aortic substitute in combination with porcine stentless aortic cusps, showing no tissue deterioration after more than 5 years from surgery (85).
A preliminary study on the usage of BPPs compared with autologous vein patches for endarterectomy of atherosclerotic carotids revealed that restenosis was uniformly present in both groups but no aneurysms were detected in case of the pericardial substitutes (86). No ruptures, aneurysmal dilatation, occlusions, and infections were reported by Grimsley et al. for implanted GA-treated BPPs at 54 months from carotid endarterectomy (87). In this surgery, BPP-based treatments demonstrated to be durable on both short and long terms (88), with a very low rate of stenosis (89,90). In the early and mid-term comparison, the performance of crosslinked PPs was similar to the one of Dacron®, PTFE, and autologous veins if re-stenosis rate and perioperative bleeding are considered (91,92). Even if generally uncommon, few exceptions of infections and aneurysmal formations have been described (93).
Another successful surgical indication for the use of GA-crosslinked APPs is the reconstruction of the pulmonary artery and superior vena cava (94), as well as angioplasty, as an alternative to bypass grafting (95).
Acellular patches
GA-treated PPs are non-viable tissues, difficultly prone to endothelialization and unlikely disposed to remodeling and regeneration for a full repopulation and integration with the recipient’s body.
Besides the countless attempts to reduce calcification potential (96-102) and cytotoxic aldehyde release from crosslinked biomaterials (103,104), new strategies aim at obtaining an improved tissue biocompatibility by means of decellularization. Ideally, decellularization procedures generate scaffolds freed from potentially immunogenic and pro-calcific cell elements, while preserving intact the properties of the ECM through the application of physical, chemical and/or enzymatic treatments (6,105). Several techniques have been proposed for pericardial tissues (6,106-109) and some of the manufactured products have already reached the market as GA-free, decellularized PPs (Table 1).
In particular, CardioCel found application for the correction of several congenital heart defects in pediatric patients, showing no evidence of calcification, infections, thickening or hemodynamic alterations on the short and long observations (110,111). Furthermore, explanted patches showed intima formation, neovascularization, and remodeling (112).
SIS-ECM patches
Preclinical experiences
A great attention has been recently addressed to the patches derived from the SIS of porcine origin. This tissue is particularly promising because of several advantages, including superior biocompatibility, manageability, time durability and potential to tissue remodeling and regeneration, with inferior proneness to scarring and calcification. For its peculiarities, SIS has immediately gained wide acceptance within the preclinical and clinical communities.
SIS derives from the jejunal portion of the porcine small intestine, being localized between the mucosal and muscular layers. In order to render it more immunocompatible, several decellularization treatments were developed (113-116). Different preparations are already commercialized as ECM scaffolds of SIS, the so-called SIS-ECMs. They consist of specific matrix proteins, as 90% collagen (predominantly type I with minor amounts of types III, IV, V and VI), fibronectin and laminin, and other non-fibrillar support structures, as glycosaminoglycans (e.g., heparan sulfate and hyaluronic acid, thought to help regulating the matrix density and inhibiting scar formation during the healing process) organized in proteoglycans, as well as growth factors and adhesion molecules able to promote a ‘constructive’ remodeling (117-122). The collagen fibers of SIS are preferably oriented along the longitudinal axis of the small intestine. This is the result of two types of collagen fibers that are aligned roughly 30° respect to the longitudinal axis of the small intestine (123,124). Probably, this particular spiral arrangement of the collagen fibers facilitates dilatation and retraction, i.e., the typical movements of the small intestine in the transport of the bolus along the digestive lumen. Understanding the alignment of ECM collagen fibers is important for the design of the ideal mechanical behavior of the scaffold and therefore of its strength.
In its acellular version, SIS has an extremely reduced immunogenic behavior (125). In addition, the bioinductive properties of the SIS-ECM confer to this scaffold an important ability of tissue remodeling. In fact, it promotes the viability of the adjacent native tissue. The high regeneration potential inherent to SIS originates from a decellularized tissue very rich in growth factors, such as vascular endothelial cell growth factor (VEGF), basic fibroblast growth factor (b-FGF), and transforming growth factor beta (TGF-β), but also owning a high biodegradability. During the scaffold degradation, a release of VEGF, b-FGF and TGF-β occurs, which induces angiogenesis, mitogenesis, and cellular differentiation in a remodeling process stimulating scar development and, thus, encouraging the development of native tissue (119). As previously described (122,124), the dynamics of the bioinduction process is not yet well deciphered but several hypotheses have been formulated, one not excluding the others. Following the first of these, ECM scaffold degradation occurs through the action of enzymes and monocytes, differentiating in macrophages with M2 phenotype rather than M1. The M2 phenotype stimulates remodeling and integration of the matrix within the tissue through the release of anti-inflammatory cytokines (IL-10 and TGF-β), while the M1 macrophage promotes the release of acidic enzymes that degrade the matrix, as well as the secretion of pro-inflammatory cytokines (126,127). Secondly, the release of growth factors, such as VEGF, TGF-β and other peptides (e.g., endostatin and angiostatin), from the degradable ECM might induce this process (124). As third, the infiltration of the recipient’s cells, including bone marrow circulating ones, might support long-term tissue remodeling (127).
CorMatrix® (CorMatrix Cardiovascular, Inc., Roswell, Atlanta, and Alpharetta, GA, USA) is one of the commercial SIS-ECM products to have found large application in cardiovascular surgery. It is marketed as a decellularized porcine SIS material, after treatment of the native tissue with proprietary techniques and following folding in four or six layers, pressed together to form a single membrane of around 100 µm-thickness thanks to the imposition of 1,200 mmHg pressure (121,128). The lyophilized CorMatrix® is available in two sizes and needs to be rehydrated (10 minutes at room temperature in a sterile saline solution) to return flexible and elastic before its application.
Since the beginning, CorMatrix® attracted the interest of scientists and clinicians for its abilities to favor migration, cell growth and differentiation thanks to its three-dimensional structure and to the growth factor content. These features promote, in turn, tissue remodeling, minimizing inflammatory responses by the recipient, as recently reviewed by Mosala Nezhad et al. (122). In particular, CorMatrix® is endowed with many characteristics of the ideal scaffold. Its biological and structural properties make it strong and durable over time.
A great number of preclinical studies on different animals evidenced the promising features for the application of SIS scaffolds in the clinical practice. Thanks to a detailed histopathological analysis, it appeared clear that an active remodeling process was undergone on porcine SIS-ECM explants, as proved by surface re-endothelialization, repopulation of the matrix with engrafted cells (fibroblasts and smooth muscle cells) and neoangiogenesis.
The first SIS study in the cardiovascular field was realized in 1989 when Badylak et al. tested the use of autologous SIS as a large diameter vascular graft (10 mm) in the canine sub-aorta model. This sensibly improved success rates in this vascular graft surgery with endothelialization of the SIS graft surface and no infection or intimal hyperplasia (125).
A study demonstrating the good perspectives of CorMatrix® cardiovascular use was provided by Mosala Nezhad et al. (129): they implanted four different biomaterials, including this commercial SIS-ECM, in the porcine subcutaneous model with a follow up of 12 months after grafting. CorMatrix® underwent a gradual and consistent resorption without leaving residues. In addition, a progressive attenuation of inflammatory and fibrotic responses was documented, facilitating cell migration and subsequent formation of a new viable tissue.
Another successful preclinical experience with this SIS-ECM was described by Padalino et al. (121). They evaluated its efficacy as a possible patch in cardiovascular reconstructive surgery, grafting CorMatrix® into the abdominal aorta in a genetically modified rodent model (GFP-transgenic rat). This study stems from the need to find a biological tissue capable of adaptation to the recipient’s somatic growth. This ability is often not possessed by other bioprostheses, rendering necessary re-do procedures during the physical development of the pediatric patient. Histopathological analyses of explanted SIS vascular grafts revealed no inflammation or calcifying degeneration, as well as significant neoangiogenetic remodeling and tissue re-endothelialization, originating from the native tissue at the patch graft site.
For the repair of the carotid artery, Fallon et al. implanted CorMatrix® (6-ply) as patch in the sheep model. The initial stenosis observed after one month completely disappeared at 90 days from grafting. In addition, a remodeling process interested the graft with SIS-ECM resorption, collagen deposition, endothelialization, neoangiogenesis, and no calcific degenerations (128).
Of all preclinical studies published on SIS-ECM, only one disclosed negative outcomes. In fact, Pavcnik et al. demonstrated a high failure rate (70%) at 3–4 months in an ovine carotid graft model due to dilation, stenosis, dissection and aneurysm formation. The authors hypothesized that the possible causes for graft failure could be related to the animal model, the relatively extended length of the graft (10 cm) and the surgical technique, as well as the response to the SIS-ECM material (130).
Clinical applications
Collagen-based connective tissue SIS-ECMs have been applied for over 20 years in the clinics for soft tissue repair and reconstruction in surgery applications in the fields of cardiovascular system (131,132), integument (133,134), body wall (135,136), urinary bladder (137,138), rotator cuff (139,140), intestine (135,141,142), urethra (143), and diaphragm (144). Besides Cormatrix®, already described in the previous section, several commercial products of acellular SIS-ECM are currently available (e.g., Surgisis®, Durasis® and Stratasis®, Cook Biotech, Lafayette, IN, USA; Restore®, DePuy, West Chester, PA, USA). A detailed list of products with information on manufacturers and pre-commercial manipulations can be retrieved in the reviews by Badylak’s group (124,126) and Scholl et al. (145).
Clinical results with Surgisis® for the treatment of carotid endarterectomy in 76 patients were reported by McCready et al.: 7 out of all patients were diagnosed with an asymptomatic pseudoaneurysm, which caused the suspension of the trial. Histopathological analysis revealed a robust presence of myofibroblasts, while biomechanical testing evidenced a strong lot-to-lot variability in the elastic properties of the product (146).
The Food and Drug Administration approved in 2010 the clinical use of Cormatrix® for cardiac and extracardiac indications. Commercialized firstly for the closure of the pericardium, its authorized application was then extended to the repair of intracardiac defect, aortic and great vessels thanks to its high manageability. To date, CorMatrix® has been applied in congenital cardiac and vascular surgery (145,147,148), pericardial reconstruction (149), intraventricular repair (150), arterial and valvular reconstruction in both adults and children, also in the case of endocarditis (151-154), acquired vascular defects at different sites (151) and repair of damaged myocardium after infarction (155). These clinical uses are all facilitated by a large scale manufacturability according to high quality control, thereby ensuring a continuous and standardized supply of material to surgeons.
However, Cormatrix® application in humans did not show the same encouraging outcomes reported in preclinical studies. Several trials have demonstrated that an intense inflammatory response was present after patch implantation. For example, in the study by Rosario-Quinones et al., 25 pediatric patients were treated with CorMatrix® patches (CorMatrix®, Atlanta, GA, USA) for repair of congenital heart malformations. Six of these grafted patients were submitted to re-do intervention and generally, all developed an intense inflammatory infiltration, prevalently of eosinophil type, as well as fibrosis. The severe eosinophilia observed in these explants was correlated to a hypersensitivity reaction to α-gal epitopes (156). These antigens are exposed on the cell surface of porcine tissues, but not on the human counterparts. Thus, this allergic response has to be attributed to the CorMatrix® itself.
In another study, Mosala Nezhad et al. used CorMatrix® for the bicuspidation of a severely stenotic unicuspid aortic valve in a 12-year-old boy. In the long-term follow up realized, CorMatrix® remained stable for 2 years. After 4 years from the grafting, the valve underwent complete calcification, fibrotic degeneration, retractions and an intense inflammatory infiltrate (157), rendering necessary a re-intervention.
Deorsola et al. applied CorMatrix® to enlarge the isthmic narrowing with the aim of restoring the structure of the original tissue. In 6 months, some stenotic complications appeared at the patch application site, subsequently re-expanded by angioplasty (158). In the case study report by Eckhauser et al., CorMatrix® SIS-ECM (CorMatrix Alpharetta, GA) was for the first time used to reconstruct a traumatic rupture of the aortoinnominate artery (also called brachiocephalic arterial trunk) of a pediatric patient in an emergency intervention. This study highlights the safety, feasibility and usage readiness of CorMatrix® in major arterial reconstruction, also in urgency medicine. However, it is important to note that there are no significant long-term data to support the benefits of this material in artery reconstruction (151).
Quarti et al. adopted CorMatrix® ECM in 26 patients for cardiac tissue repair and pericardial closure. No major, post-operative death complications or calcifications were reported but, even in this case, the follow-up length was only 25 months, so too short to evaluate the long-term functionality of this valve repair (147).
All these studies evidenced the main downside in the use of CorMatrix®, i.e., the inflammatory response, often intense and predominantly eosinophil, leading to severe stenosis and complete calcification of the patch (156,157). In addition, no reabsorption of the implanted material, minimal or totally absent tissue remodeling and negligible reconstruction of native tissues were described (158). In all cases, no systematic and long-lasting follow-up demonstrating real and proven clinical efficacy was provided.
Although some reported data are optimistic, the uncertainties on the clinical performance remain, as long as there are no large scale trials and consequent systematic follow-up. In addition, there is still a lacking consensus on robust protocols aimed at quantifying the pathological and tissue repair process, as well as the host’s immune response mechanisms towards SIS-derived patches. Nevertheless, this clinical hesitation is also related to critical ethical doubts about the trial extension to the pediatric population, given that the reoperation rate due to Cormatrix® failure is equal or even higher than 10% for this population.
Bioengineered heart tissues
Preclinical experiences
In the decades around the years 2000, an incredible ferment was generated around the possibility to clinically treat the dramatic consequences of myocardial ischemia with cell cardioplasty. In this therapeutic vision, lost cardiomyocytes could be replaced by the infusion of exogenous cells, as supported by the positive results of preclinical experiments (159-162). In the in-human translation of this concept, skeletal myoblasts, as well as stem cells of hematopoietic, mesenchymal, bone marrow and cardiac origin have been applied in the setting of acute or chronic cardiac ischemia (163-170). Generally, the outcomes of these clinical trials resulted to be not sufficient to restore heart global function, even if a minor to moderate increase of the ejection fraction was documented. The initial hypothesis of transdifferentiation of injected cells into contracting cardiomyocytes was not proved, apart for those cytotypes endowed with an original cardiac commitment. Moreover, the ischemic microenvironment characterized by inflammation, hypoxia and absence of nutrients was not ideal to maintain the viability of infused cells and/or favor their participation to healing. Paracrine effects of cell therapy were therefore the only responsible for the mild amelioration of the ischemic heart function.
The modification of the milieu became the essential step towards the target of cardiac myocardium reconstruction. The treatment paradigm shifted from the sole cell therapy to tissue re-establishment by means of cardiac tissue engineering (TE). As firstly described by Langer and Vacanti, the engineering of a tissue can be achieved by opportunely combining and stimulating cells and scaffolds in order to generate a mature and functional bioequivalent (171). First attempts with biomaterial scaffolds were performed with meshes wrapped around the ischemic ventricle to prevent its inexorable dilatation. LV restraints, developed in polypropylene, polyester and recently nitinol, demonstrated to be efficacious in reducing the end-systolic and -diastolic volumes of ischemic ventricles in animal models and clinical trials (172-174), but did not receive further attention for a routine in-human application due to the invasive cardiosurgical intervention.
The classic concept of TE was, instead, widely explored at the preclinical level. Through a multidisciplinary approach, novel solutions were designed and tested in order to offer more biocompatible and viable alternatives to so far used treatments. Scaffolds, cells and stimuli were selected among the several options on the basis of specific criterions, among which their bioactivity and non-immunogenicity. As in the case of cell therapy, cellular elements were chosen on their ability to survive, transdifferentiate into cardiac cytotypes, as well as integrate into the host tissue. Nevertheless, preferred scaffolds had to be biodegradable and easily vascularizable in order to prevent occurrences of core necrosis. First, the ability to be not seen by the recipient’s immune system, i.e., biomimesis, was also retained to be an essential property. More recently, the advances on the understanding of the response to implanted biomaterials have evidenced a peculiar role of inflammation and immunoresponse exerted by M2 macrophages in the tolerance/integration process leading to regeneration. Especially in the case of myocardial reconstruction, the in vitro integration of cells and scaffolds has to allow the establishment and maintenance of electro-mechanical coupling and pulse propagation. In order to achieve such an objective, the provision of a specific biochemical, biomechanical and electrical conditioning is usually performed with a bioreactor, able to reproduce in vitro the physiological environment of the tissue to be reconstructed.
First TE experiences faced the dramatic problem of core necrosis developing soon cell death when the thickness of manufactured tissues exceeded 100 µm. Indeed, the technology proposed by Zimmermann and Eschenhagen in 2000 revolutionized the myocardial TE world. This group advanced a novel concept of myocardial tissue construct, i.e., the engineered heart tissue, a combination of contractile rings realized in Matrigel, i.e., an immature ECM secreted by murine fibroblasts, and neonatal rat cardiac myocytes, able to develop a force superior to 2 mN following mechanical and/or chemical conditioning (175). In a further evolution of this approach, they went closer to the clinical translation by the replacement of the rodent elements with human cardiac myocytes and fibrin (176).
In the TE formulation of a functional tissue, the provision of adequate stimuli is essential but still a crucial technical issue, especially when driving differentiation of particularly immature cells. Recapitulating in a bioreactor the complex native mechanotransduction is still a difficult task although the increased knowledge in this field. The application of mechanical stimulation is essential in inducing cell alignment and force generation but needs to be precisely tuned to prevent undesired differentiation, fibrotic tissue development and/or apoptosis. A combination of neonatal cardiomyocytes and endothelial cells has demonstrated under cyclic strain to increase the alignment towards the native heart’s one, in respect to none or static conditioning (177).
As for LV restraints, not all TE strategies were devised for the in vitro reconstruction of the tissue of interest. The development of classical TE equivalents requires a relatively long process, which might be demanding to realize, as well as time-consuming due to extensive culturing in bioreactor. Hence, an associated risk of traditional TE might be the inability to obtain a mature and functional tissue substitute at the end of the conditioning. In addition, developed TE tissues could be available only for elective surgery, thus not representing a therapeutic option for patients to be urgently treated.
In order to skip this limitation, a more straightforward approach was conceived, i.e., in situ TE, combining the scaffold and/or the cells directly in the surgical theatre. With such an approach, several biomaterials alone were tested. The formulation of biomaterials available for in situ TE was very similar to the one adopted for classical strategies. In particular, hydrogels and patches in natural materials were widely used (178,179). Several types of bioconjugates were generated able to modify their state (liquid to hydrogel) in response to environmental or temperature changes, in addition to gradually release various pro-regeneration proteins. Alginate sulfate was used to create multifunctional scaffolds which, upon progressive degradation, could release drugs and/or growth factors. This was the case of the combined IGF-1/HGF hydrogel: an immediate discharge could be generated for the first growth factor to prevent cell death and increase survival, while a slower release was induced for the second one in order to enhance vascularization and prevent fibrosis (180).
Thermoresponsive polymers were also employed for a different scope, i.e., the creation of sheets of specialized cells. Synthetic materials, as poly(N-isopropylacrylamide) (PIPAAm), were applied by the Japanese group of Okano to create layers of cardiac myocytes. In a traditional culture system, cells need to be detached from their support by the enzymatic effect of trypsin in order to be further used. This treatment induces, however, the rupture of cell-cell and cell-ECM junctions, particularly essential for cardiac syncytium maintenance. Thanks to the PIPAAm technology, a modification in temperature from 37 to 20 °C is per se sufficient to cause the detachment of the entire cell layer, by inducing a variation of the polymer hydrophobic properties. As demonstrated in their long experience with PIPAAm, the mono- or multisheets of cardiomyocytes can be submitted to biochemical stimulation with growth factors, vascularized or even fashioned in tube to shape a cardiac pump, similar to a clinically implanted left ventricular assist device (LVAD) (181,182). Cell sheet technology has been realized with several cell types apart from cardiac myocytes, as for example skeletal myoblasts. Some clinical trials evidenced a risk of arrhythmia generated after cell transplantation of these cells. Not only sheets of functional skeletal myoblasts did not induce ventricular tachyarrhythmias when applied as patch, but also their therapeutic effect could be enhanced when mesenchymal stem cells were added, thanks to their pro-survival and anti-apoptotic effects (183,184).
Such an approach was also pursued to overcome the barrier of vascularization. In vivo preclinical evaluation evidenced that, in combination with omentopexy, cell sheets promoted arteriogenesis and ameliorated coronary artery perfusion, by mitigating cardiac hypertrophy and scarring (185).
Apart from thermoresponsive materials, great interest was generated by self-assembling peptides, i.e., amphiphilic molecules able to assemble into nanofibers, membranes and hydrogels in a process strictly depending, among the many variables, on the peptide sequence, concentration, pH and presence of salts. After injection, these self-assembling peptides created a suitable intramyocardial environment for the survival of endothelial cells, by downregulating apoptosis and enhancing the angiogenetic program towards a dense tridimensional (3D) net of capillaries (186,187). The possibility to decorate and biofunctionalize these peptide sequences with growth factors further increase the relevance of a potential application in humans (188).
In this panorama of novel technologies, the introduction of 3D bioprinting has allowed to prototype different cardiac tissue constructs by the aid of computational modeling. Differently from other approaches, especially of vascular reconstruction, the use of 3D bioprinters offers the opportunity to generate complex structures, as ramified arterial trees (189-191). The advantage of the easy switchable combination of cells and scaffold elements might turn to be also a drawback, since as in other TE modalities, the major difficulty is to reproduce the exact spatial distribution and cell maturation of the native tissue in the generated bioequivalent.
Among all preclinical developments in the regenerative medicine of cardiovascular organs and tissues, the adventure of induced pluripotent stem cells (iPS) has definitely signed a step forward, rendering available very plastic stem cells of adult origin able to differentiate into all cardiac lineages, e.g., cardiomyocytes, endothelial and smooth muscle cells, upon appropriate stimulation (192,193). After combination with cell sheet technology, Kawamura et al. demonstrated that generated layers of iPS-derived cardiomyocytes could be applied with successful effects in a porcine ischemic cardiomyopathy model upon combination with flap omentum (194). Moreover, Ogasawara et al. evidenced that iPS-derived cardiac myocytes co-injected with Matrigel and pro-survival elements could undergo improved engraftment and maturation, in respect with their combination with other biomaterials, as the hyaluronan-based hydrogel HyStem (195). This represents, however, an obstacle in the future clinical application of this therapy intended for the treatment of myocardial infarction: the potential clinical-grade substitute to the xenogeneic Matrigel demonstrated to possess limited ability in sustaining cell viability and differentiation and, hence, a reduced efficacy as therapeutic alternative. As another drawback, this study revealed also the formation of cartilage tissue in the cell/hydrogel injected area. A severe issue in possible iPS application is represented by the ineffective differentiation into the cells of interest, and in particular into cardiomyocytes, which, in turn, might cause lack of integration and genesis of arrhythmic foci. Okano’s group demonstrated that it is feasible to remove undifferentiated elements from fabricated cardiac cell sheets in specific culture conditions of methionine deprivation (196). Such a strategy is particularly appealing in the view of implanting beating constructs able to perfectly integrate within recipient’s cardiac tissue.
Clinical applications
The large majority of bioengineered myocardial tissues developed so far has not yet reached the clinical arena. The MAGNUM trial was one of the few studies in which the feasibility of implanting a bioartificial myocardium (collagen matrix plus bone marrow cells) was tested in 10 patients by comparison to the sole cell therapy. No mortality cases were disclosed, ascertaining the safety of the procedure (197). Results at 1 year with these implanted constructs revealed a reduction of LV telediastolic volumes and filling deceleration time, as well as an improvement in NYHA classification and ejection fraction (198).
Cell sheet technology found in-human application, too: multilayers of skeletal muscle-derived cell sheets were implanted in patients with dilated cardiomyopathy, treated with LVAD. Upon application onto the LV anterolateral surface, these engineered sheets integrated and induced angiogenesis, systolic wall thickening and regional wall contractility, allowing LVAD removal in 2 out of 4 patients (199). A larger cohort of patients was evaluated in the study of Miyagawa et al. In this phase I clinical trial, 15 and 12 patients with respectively ischemic and dilated cardiomyopathy were treated with the ventricular surface application of autologous muscle cell sheets after left thoracotomy. Patients benefited of the treatment, which in general revealed to be safe and feasible. Particularly in the case of patients with ischemic cardiomyopathy, an increase of the ejection fraction and a decrease of LV end-diastolic volume were found to be statistically significant (200). The procedure was reported to be safe also in the case of patients with severe chronic heart failure. This multicenter, phase II clinical trial evaluated the effects of muscle cell sheets in 7 severely affected cardiopatic patients, which at the end of the study revealed ameliorated NYHA class, increased LV ejection fraction and improved physical function (201).
Apart for cell-based strategies, a different therapeutic approach reached the clinical application, i.e. the use of pure biomaterials. Guided tissue regeneration with alginate has been attempted in several clinical trials. Frey et al. injected 1% sodium alginate, mixed with 0.3% calcium gluconate, through the coronary artery of 27 infarcted patients with ST-segment elevation (NCT01226563 clinical trial). Not only tissue permeation was demonstrated after 3 minutes from treatment without adverse events for coronary artery perfusion, but also procedure safety was confirmed at 6-month follow-up (202). The trial was extended internationally, enrolling a total of 303 patients, of whom 201 treated with this novel hydrogel scaffold and 102 assigned to the sham (saline injection). Although safety confirmation, no amelioration of the cardiac function could be appreciated in respect to the control group (203). In the international, multicenter, randomized AUGMENT-HF trial, a hydrogel in calcium alginate, i.e., Algisyl®, was injected in 39 patients with advanced heart failure and compared to a similar group of subjects submitted to the standard medical therapy. At 1-year follow-up, Algisyl®-treated patients presented NYHA increase and ameliorated peak VO2 (204,205). A second trial, i.e., AUGMENT-HF II (NCT03082508), is now ongoing to demonstrate safety and efficacy in an estimated enrollment of 280 subjects with heart failure.
Up to now, the few therapeutic regenerative medicine approaches reaching the clinical stage to treat heart ischemia and failure have been evaluated in small trials. It is thus imperative to enlarge the studied cohorts in order to fully evaluate the potential effect of these novel therapeutic strategies.
Bioengineered blood vessels
Preclinical experiences
The same TE concept has been applied not only for the myocardium reconstruction but also for the generation of bioengineered vessel replacements. In the last years of the previous century, the group of Mayer has started to develop novel bioequivalent solutions for both heart valves and blood vessels with the aim to overcome the limitations of non ‘living’ available prosthetic substitutes. By the combination of synthetic polymers, as polyglycolic acid (PGA) and polyhydroxyalkanoate (PHA), and ovine carotid artery cells, vascular constructs were obtained in vitro and implanted into the descending aorta of 7 lambs. While acellular polymeric tubes in control animals lost patency progressively, no aneurysmal dilatation was disclosed for TE vascular constructs, which revealed at the microscopic level formation of collagen and elastin, as well as cell population, both accountable for a biomechanical profile similar to the one of a native vessel (206). In the same years, the group also demonstrated the feasibility to generate pulmonary artery autografts starting from identical biomaterials and ovine artery- or vein-derived primary cells. The replacement of the pulmonary artery in the lamb model showed a maintained patency of the novel vessel with its progressive adaptation to the somatic growth of the animal. Although calcium content was particularly high, no gross calcifications were observed and pulmonary artery tissue appeared to be newly created, in terms of endothelialization and ECM synthesis. Also in this study, the polymeric tube used as control underwent thrombosis and loss of patency (207).
By modifying the formulation of the scaffold, Shinoka and Breuer proceeded in the evaluation of TE conduits as inferior vena cava interposition vessels. TE constructs were realized with PGA, coated with a 10% solution of L-lactide and epsilon-caprolactone, and sheep bone marrow mononuclear cells. After 6 months from implantation in 7 juvenile lambs, implanted grafts revealed physiological increase of the diameter and patency, as documented by magnetic resonance angiography. Analyzed tissue composition and ECM distribution were the typical ones characterizing a native vein, also expressing distinctive markers of normal venous formation (208). The extensive studies of the group led to hypothesize in 2010 a mechanism by which vessel tissue repopulation could occur. Following this hypothesis, the bone marrow cells, initially seeded in vitro, abandon the scaffold in favor to monocytes soon after implantation, through a program depending on the secretion of chemoattractants by the first cytotype. In turn, this is followed by the infiltration of smooth muscle actin-expressing cells and endothelialization, with monocytes leaving the tissue after scaffold degradation and ECM synthesis (209). It is fascinating that inflammatory response has been so early identified as the main actor in recipient’s tolerance and remodeling of TE constructs, even if this formulated theory is not taking into account the real prominence of the scaffold as inductive element of the entire process (210).
Despite the improved formulation, experiences with the sole scaffold revealed to be particularly disastrous in the creation of novel vessels, especially in presence of small diameters (<6 mm). Moreover, the major disadvantage of PGA use was given by the incomplete degradation of the polymeric scaffold able to sensibly modify the proliferation pattern of smooth muscle cells (211). The group of Niklason strongly contributed to the development of more biocompatible blood vessel equivalents. A new scaffold formulation in glycolide and trimethylene carbonate, eventually added with polyethylene succinate, was compared to the classic PGA, showing similar cell bioactivity, but increased induction of collagen production and improved biomechanical properties (212).
In addition, small diameter bioengineered vessels were grown in vitro by combining a natural ECM, as fibrin, with bovine smooth muscle cells and human dermal fibroblasts. Pulsatile stretch applied for 30 days on these constructs was able to induce proper synthesis of ECM main proteins, with newly secreted collagen organized in circumferential distribution. Conditioned fibrin-based vessels developed burst pressure and compliance similar to the native counterparts, as well as appropriate suturable properties (213).
More recently, this group established a novel approach of blood vessel engineering by using decellularized tissues. Acellular umbilical arteries might be used as small diameter vascular grafts thanks to the maintenance of the biomechanical and bioactive properties of their original ECM scaffolding, as demonstrated by Gui et al. after treatment with CHAPS and sodium dodecyl sulfate detergents. These human tubular scaffolds were not only prone to endothelialization in vitro, but demonstrated also to be patent for 8 weeks in vivo in a rat model of abdominal aorta interposition (214).
Clinical applications
Although in a limited number, first in-human applications have been already registered for both synthetic and decellularized arterial vessels. A tubular scaffold in PGA or in poly-L-lactic acid (PLLA) in combination with epsilon-caprolactone has been seeded with bone marrow mononuclear cells and successfully implanted in 25 pediatric patients with no aneurysmal or calcific degeneration up to 15 years, apart for 7 subjects with asymptomatic graft stenosis (215).
In an observational clinical study, Hopkins et al. used decellularized allogeneic pulmonary artery patches (MatrACELL; LifeNet Health, Inc., Virginia Beach, VA, USA) for arterioplasty in 106 patients. No degenerative events were reported during follow up, apart from restricted cases of narrowing of the juxtaductal region, without involvement of the implanted graft or stenosis. These results were particularly significant when retrospectively compared with a similar cohort of patients treated with conventional patches in synthetic material, as PTFE, and with cryopreserved pulmonary allografts (14% patch failure) (216). Interestingly, even if not properly a cardiovascular application, human decellularized vessels were effectively applied as dialysis access in 60 patients (217).
Bioengineered heart valves
Preclinical experiences
As previously anticipated, one of the first heart valve TE experiences was realized by the group of Mayer, who attempted the reconstruction of the right posterior cusp of the pulmonary valve with a PGA tissue construct seeded with fibroblasts and lined with endothelial cells. This study evidenced the feasibility of the procedure, particularly with the use of autologous cells, rather than allogeneic ones (218). Trileaflet valves in nonwoven PGA conditioned in a pulse duplicator with ovine myofibroblasts and endothelial cells for 14 days demonstrated to successfully reconstruct the right outflow tract in an autologous lamb model. In addition, the trilayered architecture of the pulmonary cusp was perfectly recreated by deposition of collagen and elastin (219).
PGA was later modified with other more biodegradable polymers, as PLLA and polyhydroxybutyrate (P4HB). Sutherland et al. constructed in vitro a valve conduit in PGA and PLLA, which was seeded with mesenchymal stem cells in a bioreactor for 1 month, prior to implantation in the juvenile sheep. Even if luminal surface endothelialization was achieved, the ECM distribution after 8 months in vivo did not reflect the anisotropic one of a mature native valve (220). With a similar valve composition and testing model, Gottlieb et al. demonstrated progressive decrease of the conduit diameter and cusp length after 20 weeks of follow up (221). The group of Hoerstrup introduced a PGA/P4HB copolymeric heart valve scaffold seeded with different cells types and adapted for several implantation modalities, as recently reviewed (222). In particular, they lately moved to the in situ heart valve TE concept, which has been adopted by different groups for ease of fabrication, tissue guided regeneration and off-the-shelf availability.
An extensive line of research in heart valve TE has been dedicated to decellularized tissues for the reconstruction of either semilunar or atrioventricular valves. As in the approach with synthetic polymers, the classical concept of TE was initially pursued to generate in vitro living heart valve substitutes (223-225), but it later became clear that the decellularized matrix had a well-defined advantage over other scaffolds, i.e. the maintenance of the original 3D architecture and matrikine cues of the native mature ECM, able to home and guide host’s cells to its repopulation (226-228). As we previously discussed (105), the choice of the decellularization formula is, hence, pivotal to profit of the benefits of a original ECM scaffold and, at the same time, to get rid of potentially immunogenic cellular components, whether the initial tissue is of allogenic or xenogeneic nature. Human leukocyte antigens (HLA), alpha-gal and/or Neu5GC sialic acids are, in fact, responsible for rejection responses in allo- and/or xenotransplantation (226,229,230). So far, the only decellularization cocktail of detergents proved to remove HLA and alpha-gal is TRICOL, i.e., a combination of osmotic shock, sodium cholate and Triton X-100 (226,230).
Clinical applications
Although several engineering strategies were applied, the retraction of the valves realized in polymeric materials represents still a sensible issue preventing their clinical application. More recently, an elastomeric valve scaffold in polycarbonate bis-urea has passed brilliantly the preclinical phase research and will be now studied in a multicenter US clinical trial (231).
The promising preclinical experience achieved during the 1990s with xenogeneic models increased the interest for an immediate clinical translation of decellularized porcine heart valves in order to reconstruct the outflow tract of pediatric patients. Clinical outcomes were catastrophic: a hyperacute rejection induced the rapid failure of these decellularized valve grafts (232), analogously to the one observed for similarly treated bovine ureteric conduits used as arterious-venous grafts in adult patients (233).
While the caution decelerated the research with xenogeneic heart valve conduits in the search for more appropriate immunologic models to evaluate tolerance at the preclinical stage, in-human studies with allogeneic valve substitutes demonstrated to be very successful with nowadays long-term observation in pediatric and adult GUCH patients (234). In the wake of these optimal outcomes, two international clinical trials ESPOIR (NCT02035540) and ARISE (NCT02527629) were launched to assess recurrence of adverse events and freedom from dysfunction in a total of 240 patients, treated with decellularized valve conduits either for right or left outflow tract reconstruction.
A multicenter trial has also recently started for the evaluation of TRICOL-decellularized allogeneic valves and preliminary results are indicative of an adequate performance during the initial follow up (unpublished data from our Centre).
Conclusions
Regenerative medicine scientists have designed several strategies in order to overcome the limitations of synthetic materials in the cardiovascular surgery of repair, correction and reconstruction of structural alterations. Shielding approaches, biomaterial fabrication, decellularization, cell reprogramming and differentiation are allowing, among the others, to biologically engineer tissue equivalents in the search for living, biocompatible and long-lasting functional replacements. Preclinical testing experiences, either in vitro or in vivo, are generally demonstrating effective cardiovascular tissue regeneration. However, the translation in the human routine therapy might find several hurdles, as first the challenging induction of immunotolerance towards the bioengineered graft. Encouraging outcomes on many TE replacements have been achieved in experimental clinical treatments but, so far, their assessment was limited to a reduced number of patients. Enlarged, multicenter clinical trials will therefore shed light on the real efficacy of these more natural surgical solutions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vaideeswar P, Mishra P, Nimbalkar M. Infective endocarditis of the Dacron patch—a report of 13 cases at autopsy. Cardiovasc Pathol 2011;20:e169-75. [Crossref] [PubMed]
- Robinson KA, Li J, Mathison M, et al. Extracellular matrix scaffold for cardiac repair. Circulation 2005;112:I135-43. [PubMed]
- Ionescu MI, Pakrashi BC, Holden MP, et al. Results of aortic valve replacement with frame-supported fascia lata and pericardial grafts. J Thorac Cardiovasc Surg 1972;64:340-53. [PubMed]
- Ionescu MI, Tandon AP, Mary DA, et al. Heart valve replacement with the Ionescu-Shiley pericardial xenograft. J Thorac Cardiovasc Surg 1977;73:31-42. [PubMed]
- Umashankar PR, Kumari T, Mohanan P. Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol Int 2012;19:51. [Crossref] [PubMed]
- Aguiari P, Iop L, Favaretto F, et al. In vitro comparative assessment of decellularized bovine pericardial patches and commercial bioprosthetic heart valves. Biomed Mater 2017;12. [Crossref] [PubMed]
- Gallo JI, Pomar JL, Artiñano E, et al. Heterologous Pericardium for the Closure of Pericardial Defects. Ann Thorac Surg 1978;26:149-54. [Crossref] [PubMed]
- Gabbay S, Bortolotti U, Factor S, et al. Calcification of implanted xenograft pericardium. Influence of site and function. J Thorac Cardiovasc Surg 1984;87:782-7. [PubMed]
- Pires AC, Saporito WF, Cardoso SH, et al. Bovine pericardium used as a cardiovascular patch. Heart Surg Forum 1999;2:60-9. [PubMed]
- Kadowaki MH, Levett JM, Manjoney DL, et al. Comparison of prosthetic graft materials as intracardiac right atrial patches. J Surg Res 1986;41:65-74. [Crossref] [PubMed]
- Kadowaki MH, Levett JM, Manjoney DL, et al. Comparative studies of prosthetic materials in the left atrium of the dog. Virchows Arch A Pathol Anat Histopathol 1987;411:173-7. [Crossref] [PubMed]
- Gabbay S, Guindy AM, Andrews JF, et al. New outlook on pericardial substitution after open heart operations. Ann Thorac Surg 1989;48:803-12. [Crossref] [PubMed]
- Meus PJ, Wernly JA, Campbell CD, et al. Long-term evaluation of pericardial substitutes. J Thorac Cardiovasc Surg 1983;85:54-8. [PubMed]
- Golomb G, Schoen FJ, Smith MS, et al. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol 1987;127:122-30. [PubMed]
- Liao K, Frater RWM, LaPietra A, et al. Time-dependent effect of glutaraldehyde on the tendency to calcify of both autografts and xenografts. Ann Thorac Surg 1995;60:S343-7. [Crossref] [PubMed]
- Salgaller ML, Bajpai PK. Immunogenicity of glutaraldehyde-treated bovine pericardial tissue xenografts in rabbits. J Biomed Mater Res 1985;19:1-12. [Crossref] [PubMed]
- Dahm M, Lyman WD, Factor SM, et al. Immunogenicity of Glutaraldehyde-Tanned Bovine Pericardium. In: Cardiac Reconstructions. Berlin, Heidelberg: Springer Berlin Heidelberg; 1989:360-6.
- Admedus. CardioCel. Available online: https://www.admedus.com
- Edwards. Bovine Pericardial Patch. Available online: http://www.edwards.com
- Auto Tissue Berlin GmbH. Matrix PatchTM. Available online: http://www.autotissue.de
- BioIntegral Surgical Inc. No-React Patch. Available online: https://www.biointegral-surgical.com
- Baxter. Peri-Guard. Available online: http://ecatalog.baxter.com
- Neovasc Inc. News Release - Neovasc Inc. Receives CE Mark for Peripatch-BV Bovine Pericardial Tiussue. Available online: http://www.neovasc.com
- CryoLife. PhotoFix Decellularized Bovine Pericardium. Available online: https://www.cryolife.com
- . Porcine Pericardial Patch. Available online: http://oem.vascutek.comVascutek OEM.
- St. Jude Medical. SJM BiocorTM Patch Bovine Pericardium Repair Patch. Available online: https://www.sjmglobal.com
- St. Jude Medical. SJMTM Pericardial Patch with EnCapTM AC Technology. Available online: https://www.sjm.com
- FOC Medical. Bovine Pericardium Patches SURGIFOC. Available online: http://focmedical.com
- Tissue Regenix Group PLC. dCELL vascular patch. Available online: https://www.tissueregenix.com
- Baxter. Vascu-Guard. Available online: http://ecatalog.baxter.com
- Nandi P, Leung JS, Cheung KL. Closure of pericardium after open heart surgery. A way to prevent postoperative cardiac tamponade. Br Heart J 1976;38:1319-23. [Crossref] [PubMed]
- Youmans CR Jr, White J, Derrick JR. The prevention of pleural and pericardial adhesions with Silastic. J Thorac Cardiovasc Surg 1968;55:383-8. [PubMed]
- Mazuji MK, Lett JC. Siliconized Dacron as a Pericardial Patch. Arch Surg 1963;87:446-9. [Crossref] [PubMed]
- Bonnabeau RC, Armanious AW, Tarnay TJ. Partial replacement of pericardium with dura substitute. J Thorac Cardiovasc Surg 1973;66:196-201. [PubMed]
- Laks H, Hammond G, Geha AS. Use of silicone rubber as a pericardial substitute to facilitate reoperation in cardiac surgery. J Thorac Cardiovasc Surg 1981;82:88-92. [PubMed]
- Mestres CA, Comas JV, S. N, et al. The use of polyetherurethane urea (Mitrathane) and polytetrafluoroethylene (Gore-Tex) membrane for pericardial closure: initial clinical results. Thai J Surg 1986:125-8.
- Revuelta JM, Garcia-Rinaldi R, Val F, et al. Expanded polytetrafluoroethylene surgical membrane for pericardial closure. An experimental study. J Thorac Cardiovasc Surg 1985;89:451-5. [PubMed]
- Minale C, Hollweg G, Nikol S, et al. Closure of the pericardium using expanded polytetrafluoroethylene GORE-TEX®-surgical membrane: Clinical experience. Thorac Cardiovasc Surg 1987;35:312-5. [Crossref] [PubMed]
- Schechter FG, Owens RR, Bryant LR. Pleural Flap Closure of Pericardial Defects Following Intrapericardial Pneumonectomy. Ann Thorac Surg 1976;21:67-9. [Crossref] [PubMed]
- Kohanna FH, Adams PX, Cunningham JN, et al. Use of autologous fascia lata as a pericardial substitute following open-heart surgery. J Thorac Cardiovasc Surg 1977;74:14-9. [PubMed]
- Kageyama Y, Suzuki K, Matsushita K, et al. Pericardial closure using fascia lata in patients undergoing pneumonectomy with pericardiectomy. Ann Thorac Surg 1998;66:586-7. [Crossref] [PubMed]
- Ku YC. The repair of operative pericardial defect with fascia lata for the prevention and treatment of herniation of the heart. Chin Med J 1959;78:210-3. [PubMed]
- Gallo I, Artiñano E, Duran CG. Late clinical results with the use of heterologous pericardium for closure of the pericardial cavity. J Thorac Cardiovasc Surg 1985;89:709-12. [PubMed]
- Yakirevich VS, Abdulali SA, Abbott CR, et al. Reconstruction of the pericardial sac with glutaraldehyde-preserved bovine pericardium. Tex Heart Inst J 1984;11:238-42. [PubMed]
- Skinner JR, Kim H, Toon RS, et al. Inflammatory epicardial reaction to processed bovine pericardium: case report. J Thorac Cardiovasc Surg 1984;88:789-91. [PubMed]
- Mills SA. Complications associated with the use of heterologous bovine pericardium for pericardial closure. J Thorac Cardiovasc Surg 1986;92:446-9. [PubMed]
- von Segesser L, Jornod N, Faidutti B. Repeat sternotomy after reconstruction of the pericardial sac with glutaraldehyde-preserved equine pericardium. J Thorac Cardiovasc Surg 1987;93:616-9. [PubMed]
- Seybold-Epting W, Chiariello L, Hallman GL, et al. Aneurysm of Pericardial Right Ventricular Outflow Tract Patches. Ann Thorac Surg 1977;24:237-40. [Crossref] [PubMed]
- Graham TP, Cordell D, Atwood GF, et al. Right ventricular volume characteristics before and after palliative and reparative operation in tetralogy of Fallot. Circulation 1976;54:417-23. [Crossref] [PubMed]
- Hawe A, Rastelli GC, Ritter DG, et al. Management of right ventricular outflow tract in severe tetralogy of Fallot. Am J Cardiol 1969;23:118.. [Crossref]
- Butera G, Aggoun Y, Bonnet D, et al. Aneurysmal dilation of a pericardial patch prepared with glutharaldehyde and used for closure of a ventricular septal defect. Ital Heart J 2001;2:317-8. [PubMed]
- Bennink GBW., Hitchcock FJ, Molenschot M, et al. Aneurysmal pericardial patch producing right ventricular inflow obstruction. Ann Thorac Surg 2001;71:1346-7. [Crossref] [PubMed]
- Okwulehie V, Dharmapuram AK, Swain SK, et al. Experience with autologous pericardial patch closure of ventricular septal defect. Indian J Thorac Cardiovasc Surg 2006;22:212-4. [Crossref]
- Schoof PH, Hazekamp MG, van Ulzen K, et al. Autologous pericardium for ventricular septal defect closure. J Heart Valve Dis 1998;7:407-9. [PubMed]
- Crawford FA, Sade RM, Spinale F. Bovine Pericardium for Correction of Congenital Heart Defects. Ann Thorac Surg 1986;41:602-5. [Crossref] [PubMed]
- Luk A, Ahn E, Soor GS, et al. Pericardial patch repair of the left atrioventricular valve in atrioventricular septal defect: long-term changes in the patch. Cardiovasc Pathol 2009;18:119-22. [Crossref] [PubMed]
- Morell VO, Wearden PA. Experience With Bovine Pericardium for the Reconstruction of the Aortic Arch in Patients Undergoing a Norwood Procedure. Ann Thorac Surg 2007;84:1312-5. [Crossref] [PubMed]
- van Son JA, Kinzel P, Mohr FW. Pericardial patch augmentation of anterior tricuspid leaflet in Ebstein’s anomaly. Ann Thorac Surg 1998;66:1831-2. [Crossref] [PubMed]
- Barbero-Marcial M, Tamanati C, Jatene MB, et al. Double-outlet right ventricle with nonrelated ventricular septal defect: surgical results using the multiple patches technique. Heart Surg Forum 1998;1:125-9. [PubMed]
- Barbero-Marcial M, Tanamati C, Atik E, et al. Intraventricular repair of double-outlet right ventricle with noncommitted ventricular septal defect: Advantages of multiple patches. J Thorac Cardiovasc Surg 1999;118:1056-67. [Crossref] [PubMed]
- Crawford FA. Repair of complete atrioventricular septal defects “single patch” technique. Oper Tech Thorac Cardiovasc Surg 2004;9:221-32. [Crossref]
- Daebritz SH. Correction of complete atrioventricular septal defects with two patch technique. Oper Tech Thorac Cardiovasc Surg 2004;9:208-20. [Crossref]
- Doss M, Moid R, Wood JP, et al. Pericardial patch augmentation for reconstruction of incompetent bicuspid aortic valves. Ann Thorac Surg 2005;80:304-7. [Crossref] [PubMed]
- Lausberg HF, Aicher D, Langer F, et al. Aortic valve repair with autologous pericardial patch. Eur J Cardiothorac Surg 2006;30:244-9. [Crossref] [PubMed]
- d’Udekem Y, Siddiqui J, Seaman CS, et al. Long-term results of a strategy of aortic valve repair in the pediatric population. J Thorac Cardiovasc Surg 2013;145:461-7. [Crossref] [PubMed]
- Celiento M, Saccocci M, De Martino A, et al. Stability of aortic annulus enlargement during aortic valve replacement using a bovine pericardial patch: An 18-year clinical, echocardiographic, and angio-computed tomographic follow-up. J Thorac Cardiovasc Surg 2014;147:977-83. [Crossref] [PubMed]
- Milgalter E, Laks H. Use of a pericardial patch to bridge the conduction tissue during tricuspid valve replacement. Ann Thorac Surg 1991;52:1337-9. [Crossref] [PubMed]
- Arena V, Alamanni F, Repossini A, et al. Straddling endoventricular pericardial patch in prevention of type I myocardial rupture. Ann Thorac Surg 1993;56:163-5. [Crossref] [PubMed]
- Balkanay M, Eren E, Keles C, et al. Double-patch repair of postinfarction ventricular septal defect. Tex Heart Inst J 2005;32:43-6. [PubMed]
- Imagawa H, Takano S, Shiozaki T, et al. Two-Patch Technique for Postinfarction Inferoposterior Ventricular Septal Defect. Ann Thorac Surg 2009;88:692-4. [Crossref] [PubMed]
- Fiore AC, McKeown PP, Misbach GA, et al. The Use of Autologous Pericardium for Ventricular Aneurysm Closure. Ann Thorac Surg 1988;45:570-1. [Crossref] [PubMed]
- Iguidbashian JP, Follette DM, Contino JP, et al. Pericardial patch repair of left ventricular aneurysm. Ann Thorac Surg 1993;55:1022-4. [Crossref] [PubMed]
- Suzer K, Yorgancioglu C, Gunaydin S, et al. Surgical Management of Left Ventricular Aneurysms by Endoventricular Pericardial Patch Plasty. Cardiovasc Surg 2002;10:216-21. [Crossref] [PubMed]
- David TE, Feindel CM, Ropchan G V. Reconstruction of the left ventricle with autologous pericardium. J Thorac Cardiovasc Surg 1987;94:710-4. [PubMed]
- McMillan WD, Leville CD, Hile CN. Bovine pericardial patch repair in infected fields. J Vasc Surg 2012;55:1712-5. [Crossref] [PubMed]
- Garcia Aroz S, Spaggiari M, Jeon H, et al. The use of bovine pericardial patch for vascular reconstruction in infected fields for transplant recipients. J Vasc Surg Cases Innov Tech 2017;3:47-9. [Crossref] [PubMed]
- Mayer K, Aicher D, Feldner S, et al. Repair versus replacement of the aortic valve in active infective endocarditis. Eur J Cardiothorac Surg 2012;42:122-7. [Crossref] [PubMed]
- Evans CF, DeFilippi CR, Shang E, et al. Fresh autologous pericardium for leaflet perforation repair in mitral valve infective endocarditis. J Heart Valve Dis 2013;22:560-6. [PubMed]
- Marien BJ, Raffetto JD, Seidman CS, et al. Bovine Pericardium vs Dacron for Patch Angioplasty After Carotid Endarterectomy. Arch Surg 2002;137:785-8. [Crossref] [PubMed]
- Speer DP, Chvapil M, Eskelson CD, et al. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res 1980;14:753-64. [Crossref] [PubMed]
- Eybl E, Griesmacher A, Grimm M, et al. Toxic effects of aldehydes released from fixed pericardium on bovine aortic endothelial cells. J Biomed Mater Res 1989;23:1355-65. [Crossref] [PubMed]
- Huang-Lee LL, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res 1990;24:1185-201. [Crossref] [PubMed]
- Wiebe D, Megerman J, L’Italien GJ, et al. Glutaraldehyde release from vascular prostheses of biologic origin. Surgery 1988;104:26-33. [PubMed]
- Gontijo B, Sobrinho ALO, Fantini E, et al. Replacement of the ascending aorta and aortic arch with bovine pericardial grafts. A preliminary report. Eur J Cardiothorac Surg 1995;9:127-32. [Crossref] [PubMed]
- Vrandecic M, Gontijo Filho B, Fantini F, et al. Use of bovine pericardial tissue for aortic valve and aortic root replacement: long-term results. J Heart Valve Dis 1998;7:195-201. [PubMed]
- Kim GE, Kwon TW, Cho YP, et al. Carotid Endarterectomy with Bovine Patch Angioplasty: A Preliminary Report. Cardiovasc Surg 2001;9:458-62. [Crossref] [PubMed]
- Grimsley BR, Wells JK, Pearl GJ, et al. Bovine pericardial patch angioplasty in carotid endarterectomy. Am Surg 2001;67:890-5. [PubMed]
- Papakostas JC, Avgos S, Arnaoutoglou E, et al. Use of the vascu-guard bovine pericardium patch for arteriotomy closure in carotid endarterectomy. Early and long-term results. Ann Vasc Surg 2014;28:1213-8. [Crossref] [PubMed]
- Biasi GM, Sternjakob S, Mingazzini PM, et al. Nine-year experience of bovine pericardium patch angioplasty during carotid endarterectomy. J Vasc Surg 2002;36:271-7. [Crossref] [PubMed]
- Neuhauser B, Oldenburg WA. Polyester vs. Bovine Pericardial Patching during Carotid Endarterectomy: Early Neurologic Events and Incidence of Restenosis. Cardiovasc Surg 2003;11:465-70. [Crossref] [PubMed]
- Hines GL, Feuerman M, Cappello D, et al. Results of Carotid Endarterectomy with Pericardial Patch Angioplasty: Rate and Predictors of Restenosis. Ann Vasc Surg 2007;21:767-71. [Crossref] [PubMed]
- Ho KJ, Nguyen LL, Menard MT. Intermediate-term outcome of carotid endarterectomy with bovine pericardial patch closure compared with Dacron patch and primary closure. J Vasc Surg 2012;55:708-14. [Crossref] [PubMed]
- Moghadam SP, Kumar S, Fisher RK, et al. Carotid artery pseudoaneurysm after carotid endarterectomy and bovine pericardial patch angioplasty: Case report. EJVES Extra 2011;22:e67-9. [Crossref]
- D’Andrilli A, Ibrahim M, Venuta F, et al. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005;80:357-8. [Crossref] [PubMed]
- Harling L, Sepehripour AH, Ashrafian H, et al. Surgical patch angioplasty of the left main coronary artery. Eur J Cardiothorac Surg 2012;42:719-27. [Crossref] [PubMed]
- Sucu N, Tamer L, Aytacoglu B, et al. Inhibition of calcification with citric acid in pericardial bioprosthetic heart valve material: a preliminary report. J Heart Valve Dis 2004;13:697-700. [PubMed]
- Sucu N, Tamer L, Dondas HA, et al. The effect of ethylenediaminetetraacetic acid on calcific degeneration in bovine pericardium. Heart Vessels 2004;19:89-93. [Crossref] [PubMed]
- Pettenazzo E, Valente M, Thiene G. Octanediol treatment of glutaraldehyde fixed bovine pericardium: evidence of anticalcification efficacy in the subcutaneous rat model. Eur J Cardiothorac Surg 2008;34:418-22. [Crossref] [PubMed]
- Guldner NW, Jasmund I, Zimmermann H, et al. Detoxification and endothelialization of glutaraldehyde-fixed bovine pericardium with titanium coating a new technology for cardiovascular tissue engineering. Circulation 2009;119:1653-60. [Crossref] [PubMed]
- Lee C, Kim SH, Choi SH, et al. High-concentration glutaraldehyde fixation of bovine pericardium in organic solvent and post-fixation glycine treatment: In vitro material assessment and in vivo anticalcification effect. Eur J Cardiothorac Surg 2011;39:381-7. [Crossref] [PubMed]
- Jeong S, Yoon EJ, Lim HG, et al. The Effect of Space Fillers in the Cross-Linking Processes of Bioprosthesis. Biores Open Access 2013;2:98-106.
- Lee WK, Park KD, Kim YH, et al. Improved calcification resistance and biocompatibility of tissue patch grafted with sulfonated PEO or heparin after glutaraldehyde fixation. J Biomed Mater Res 2001;58:27-35. [Crossref] [PubMed]
- Moritz A, Grimm M, Eybl E, et al. Improved spontaneous endothelialization by postfixation treatment of bovine pericardium. Eur J Cardiothorac Surg 1991;5:155-9. [Crossref] [PubMed]
- Moritz A, Grimm M, Eybl E, et al. Improved endothelialization of postfixation treated biological vascular grafts. Int J Artif Organs 1992;15:289-94. [Crossref] [PubMed]
- Iop L, Gerosa G. Guided Tissue Regeneration in Heart Valve Replacement: From Preclinical Research to First-in-Human Trials. Biomed Res Int 2015;2015. [Crossref] [PubMed]
- Mirsadraee S, Wilcox HE, Korossis SA, et al. Development and Characterization of an Acellular Human Pericardial Matrix for Tissue Engineering. Tissue Eng 2006;12:763-73. [Crossref] [PubMed]
- Hülsmann J, Grün K, El Amouri S, et al. Transplantation material bovine pericardium: Biomechanical and immunogenic characteristics after decellularization vs. glutaraldehyde-fixing. Xenotransplantation 2012;19:286-97. [Crossref] [PubMed]
- Min BJ, Kim YJ, Choi JW, et al. Histologic Characteristics and Mechanical Properties of Bovine Pericardium Treated with Decellularization and α-Galactosidase: A Comparative Study. Korean J Thorac Cardiovasc Surg 2012;45:368-79. [Crossref] [PubMed]
- Fidalgo C, Iop L, Sciro M, et al. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater 2018;67:282-94. [Crossref] [PubMed]
- Neethling WML, Strange G, Firth L, et al. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: Initial experience with the ADAPT-treated CardioCel® patch. Interact Cardiovasc Thorac Surg 2013;17:698-702. [Crossref] [PubMed]
- Sobieraj M, Cudak E, Mrówczyński W, et al. Application of the CardioCel bovine pericardial patch - a preliminary report. Kardiochir Torakochirurgia Pol 2016;13:210-2. [Crossref] [PubMed]
- Prabhu S, Armes JE, Bell D, et al. Histologic Evaluation of Explanted Tissue-Engineered Bovine Pericardium (CardioCel). Semin Thorac Cardiovasc Surg 2017;29:356-63. [Crossref] [PubMed]
- Hodde JP, Record RD, Tullius RS, et al. Retention of Endothelial Cell Adherence to Porcine-Derived Extracellular Matrix after Disinfection and Sterilization. Tissue Eng 2002;8:225-34. [Crossref] [PubMed]
- Luo JC, Chen W, Chen XH, et al. A multi-step method for preparation of porcine small intestinal submucosa (SIS). Biomaterials 2011;32:706-13. [Crossref] [PubMed]
- Andrée B, Bär A, Haverich A, et al. Small intestinal submucosa segments as matrix for tissue engineering Tissue Eng Part B Rev 2013;19:279-91. review. [Crossref] [PubMed]
- Syed O, Walters NJ, Day RM, et al. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater 2014;10:5043-54. [Crossref] [PubMed]
- Hodde JP, Badylak SF, Brightman AO, et al. Glycosaminoglycan Content of Small Intestinal Submucosa: A Bioscaffold for Tissue Replacement. Tissue Eng 1996;2:209-17. [Crossref] [PubMed]
- Chen MK, Badylak SF. Small Bowel Tissue Engineering Using Small Intestinal Submucosa as a Scaffold. J Surg Res 2001;99:352-358. [Crossref] [PubMed]
- Hodde JP, Record RD, Liang HA, et al. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium 2001;8:11-24. [Crossref] [PubMed]
- Lindberg K, Badylak SF. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns 2001;27:254-266. [Crossref] [PubMed]
- Padalino MA, Castellani C, Dedja A, et al. Extracellular matrix graft for vascular reconstructive surgery: evidence of autologous regeneration of the neoaorta in a murine model. Eur J Cardiothorac Surg 2012;42:e128-35. [Crossref] [PubMed]
- Mosala Nezhad Z, Poncelet A, de Kerchove L, et al. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: a systematic review. Interact Cardiovasc Thorac Surg 2016;22:839-50. [Crossref] [PubMed]
- Sacks MS, Gloeckner DC. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res 1999;46:1-10. [Crossref] [PubMed]
- Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28:3587-93. [Crossref] [PubMed]
- Badylak SF, Lantz GC, Coffey A, et al. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 1989;47:74-80. [Crossref] [PubMed]
- Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol 2008;20:109-16. [Crossref] [PubMed]
- Piterina AV, Cloonan A, Meaney C, et al. ECM-Based Materials in Cardiovascular Applications: Inherent Healing Potential and Augmentation of Native Regenerative Processes. Int J Mol Sci 2009;10:4375-417. [Crossref] [PubMed]
- Fallon A, Goodchild T, Wang R, et al. Remodeling of Extracellular Matrix Patch used for Carotid Artery Repair. J Surg Res 2012;175:e25-34. [Crossref] [PubMed]
- Mosala Nezhad Z, Poncelet A, Fervaille C, et al. Comparing the Host Reaction to CorMatrix and Different Cardiac Patch Materials Implanted Subcutaneously in Growing Pigs. Thorac Cardiovasc Surg 2017. [Epub ahead of print]. [PubMed]
- Pavcnik D, Obermiller J, Uchida BT, et al. Angiographic Evaluation of Carotid Artery Grafting with Prefabricated Small-Diameter, Small-Intestinal Submucosa Grafts in Sheep. Cardiovasc Intervent Radiol 2009;32:106-13. [Crossref] [PubMed]
- Lantz GC, Badylak SF, Hiles MC, et al. Small Intestinal Submucosa as a Vascular Graft: A Review. J Invest Surg 1993;6:297-310. [Crossref] [PubMed]
- Holubec T, Caliskan E, Sündermann SH, et al. The Use of Extracellular Matrix Patches in Cardiac Surgery. J Card Surg 2015;30:145-8. [Crossref] [PubMed]
- Zhang F, Zhu C, Oswald T, et al. Porcine Small Intestinal Submucosa as a Carrier for Skin Flap Prefabrication. Ann Plast Surg 2003;51:488-92. [Crossref] [PubMed]
- MacLeod TM, Sarathchandra P, Williams G, et al. Evaluation of a porcine origin acellular dermal matrix and small intestinal submucosa as dermal replacements in preventing secondary skin graft contraction. Burns 2004;30:431-7. [Crossref] [PubMed]
- Helton WS, Fisichella PM, Berger R, et al. Short-term Outcomes With Small Intestinal Submucosa for Ventral Abdominal Hernia. Arch Surg 2005;140:549. [Crossref] [PubMed]
- Puccio F, Solazzo M, Marciano P. Comparison of three different mesh materials in tension-free inguinal hernia repair: prolene versus Vypro versus surgisis. Int Surg 2005;90:S21-3. [PubMed]
- Alpert SA, Cheng EY, Kaplan WE, et al. Bladder neck fistula after the complete primary repair of exstrophy: a multi-institutional experience. J Urol 2005;174:1687-89; discussion 1689-90.
- Misseri R, Cain MP, Casale AJ, et al. Small intestinal submucosa bladder neck slings for incontinence associated with neuropathic bladder. J Urol 2005;174:1680-2; discussion 1682.
- Sclamberg SG, Tibone JE, Itamura JM, et al. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg 2004;13:538-41. [Crossref] [PubMed]
- Malcarney HL, Bonar F, Murrell GAC. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med 2005;33:907-11. [Crossref] [PubMed]
- Sardeli C, Axelsen SM, Bek KM. Use of porcine small intestinal submucosa in the surgical treatment of recurrent rectocele in a patient with Ehlers–Danlos syndrome type III. Int Urogynecol J Pelvic Floor Dysfunct 2005;16:504-5. [Crossref] [PubMed]
- Hussey GS, Cramer MC, Badylak SF. Extracellular Matrix Bioscaffolds for Building Gastrointestinal Tissue. Cell Mol Gastroenterol Hepatol 2017;5:1-13. [Crossref] [PubMed]
- Jones JS, Vasavada SP, Abdelmalak JB, et al. Sling may hasten return of continence after radical prostatectomy. Urology 2005;65:1163-7. [Crossref] [PubMed]
- Oelschlager BK, Barreca M, Chang L, et al. The use of small intestine submucosa in the repair of paraesophageal hernias: initial observations of a new technique. Am J Surg 2003;186:4-8. [Crossref] [PubMed]
- Scholl FG, Boucek MM, Chan KC, et al. Preliminary Experience With Cardiac Reconstruction Using Decellularized Porcine Extracellular Matrix Scaffold. World J Pediatr Congenit Heart Surg 2010;1:132-6. [Crossref] [PubMed]
- McCready RA, Hodde J, Irwin RJ, et al. Pseudoaneurysm formation in a subset of patients with small intestinal submucosa biologic patches after carotid endarterectomy. J Vasc Surg 2005;41:782-8. [Crossref] [PubMed]
- Quarti A, Nardone S, Colaneri M, et al. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact Cardiovasc Thorac Surg 2011;13:569-72. [Crossref] [PubMed]
- Witt RG, Raff G, Van Gundy J, et al. Short-term experience of porcine small intestinal submucosa patches in paediatric cardiovascular surgery. Eur J Cardiothorac Surg 2013;44:72-6. [Crossref] [PubMed]
- Boyd WD, Johnson WE, Sultan PK, et al. Pericardial reconstruction using an extracellular matrix implant correlates with reduced risk of postoperative atrial fibrillation in coronary artery bypass surgery patients. Heart Surg Forum 2010;13:E311-6. [Crossref] [PubMed]
- Yanagawa B, Rao V, Yau TM, et al. Initial Experience With Intraventricular Repair Using CorMatrix Extracellular Matrix. Innovations (Phila) 2013;8:348-52. [Crossref] [PubMed]
- Eckhauser AW, Hannon D, Molitor M, et al. Repair of Traumatic Aortoinnominate Disruption Using CorMatrix. Ann Thorac Surg 2013;95:e99-e101. [Crossref] [PubMed]
- Cua CL, Kollins K, McConnell PI. Echocardiographic Analysis of an Extracellular Matrix Tricuspid Valve. Echocardiography 2014;31:E264-6. [Crossref] [PubMed]
- Wallen J, Rao V. Extensive tricuspid valve repair after endocarditis using CorMatrix extracellular matrix. Ann Thorac Surg 2014;97:1048-50. [Crossref] [PubMed]
- Gerdisch MW, Boyd WD, Harlan JL, et al. Early experience treating tricuspid valve endocarditis with a novel extracellular matrix cylinder reconstruction. J Thorac Cardiovasc Surg 2014;148:3042-8. [Crossref] [PubMed]
- Yanagawa B, Rao V, Yau TM, et al. Potential myocardial regeneration with CorMatrix ECM: a case report. J Thorac Cardiovasc Surg 2014;147:e41-3. [Crossref] [PubMed]
- Rosario-Quinones F, Magid MS, Yau J, et al. Tissue reaction to porcine intestinal Submucosa (CorMatrix) implants in pediatric cardiac patients: a single-center experience. Ann Thorac Surg 2015;99:1373-7. [Crossref] [PubMed]
- Mosala Nezhad Z, Baldin P, Poncelet A, et al. Calcific Degeneration of CorMatrix 4 Years After Bicuspidization of Unicuspid Aortic Valve. Ann Thorac Surg 2017;104:e431-3. [Crossref] [PubMed]
- Deorsola L, Pace Napoleone C, Abbruzzese PA. Repair of an unusual aortic coarctation using an extracellular matrix patch. Ann Thorac Surg 2014;97:1059-61. [Crossref] [PubMed]
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5. [Crossref] [PubMed]
- Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 2002;73:1919-25; discussion 1926.
- Hu X, Wang J, Chen J, et al. Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. Eur J Cardiothorac Surg 2007;31:438-43. [Crossref] [PubMed]
- Iop L, Chiavegato A, Callegari A, et al. Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury. Cell Transplant 2008;17:679-94. [Crossref] [PubMed]
- Farahmand P, Lai TY, Weisel RD, et al. Skeletal Myoblasts Preserve Remote Matrix Architecture and Global Function When Implanted Early or Late After Coronary Ligation Into Infarcted or Remote Myocardium. Circulation 2008;118:S130-7. [Crossref] [PubMed]
- Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369-79. [Crossref] [PubMed]
- Zimmet H, Porapakkham P, Porapakkham P, et al. Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail 2012;14:91-105. [Crossref] [PubMed]
- Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: The C-CURE (cardiopoietic stem cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 2013;61:2329-38. [Crossref] [PubMed]
- Malliaras K, Makkar RR, Smith RR, et al. Intracoronary Cardiosphere-Derived Cells After Myocardial Infarction. J Am Coll Cardiol 2014;63:110-22. [Crossref] [PubMed]
- Sanz-Ruiz R, Casado Plasencia A, Borlado LR, et al. Rationale and Design of a Clinical Trial to Evaluate the Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With Acute Myocardial Infarction and Left Ventricular DysfunctionNovelty and Significance. Circ Res 2017;121:71-80. [Crossref] [PubMed]
- Ishigami S, Ohtsuki S, Eitoku T, et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle PhysiologyNovelty and Significance. Circ Res 2017;120:1162-73. [Crossref] [PubMed]
- Chakravarty T, Makkar RR, Ascheim DD, et al. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) Trial: Rationale and Design. Cell Transplant 2017;26:205-14. [Crossref] [PubMed]
- Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920-6. [Crossref] [PubMed]
- Kelley ST, Malekan R, Gorman JH, et al. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation 1999;99:135-142. [Crossref] [PubMed]
- Konertz WF, Shapland JE, Hotz H, et al. Passive containment and reverse remodeling by a novel textile cardiac support device. Circulation 2001;104:I270-5. [Crossref] [PubMed]
- Sabbah HN, Sharov VG, Gupta RC, et al. Reversal of Chronic Molecular and Cellular Abnormalities Due to Heart Failure by Passive Mechanical Ventricular Containment. Circ Res 2003;93:1095-101. [Crossref] [PubMed]
- Zimmermann WH, Fink C, Kralisch D, et al. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng 2000;68:106-14. [Crossref] [PubMed]
- Breckwoldt K, Letuffe-Brenière D, Mannhardt I, et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat Protoc 2017;12:1177-97. [Crossref] [PubMed]
- Tulloch NL, Muskheli V, Razumova M V, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 2011;109:47-59. [Crossref] [PubMed]
- Wang H, Zhang X, Li Y, et al. Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive chitosan hydrogel. J Heart Lung Transplant 2010;29:881-7. [Crossref] [PubMed]
- Callegari A, Bollini S, Iop L, et al. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 2007;28:5449-61. [Crossref] [PubMed]
- Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011;32:565-578. [Crossref] [PubMed]
- Sekine H, Shimizu T, Sakaguchi K, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun 2013;4:1399. [Crossref] [PubMed]
- Masuda S, Shimizu T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv Drug Deliv Rev 2016;96:103-9. [Crossref] [PubMed]
- Terajima Y, Shimizu T, Tsuruyama S, et al. Autologous Skeletal Myoblast Sheet Therapy for Porcine Myocardial Infarction without Increasing Risk of Arrhythmia. Cell Med 2013;6:99-109. [Crossref] [PubMed]
- Shudo Y, Miyagawa S, Ohkura H, et al. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng Part A 2014;20:728-39. [PubMed]
- Kainuma S, Miyagawa S, Fukushima S, et al. Cell-sheet Therapy With Omentopexy Promotes Arteriogenesis and Improves Coronary Circulation Physiology in Failing Heart. Mol Ther 2015;23:374-86. [Crossref] [PubMed]
- Davis ME, Motion JP, Narmoneva DA, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 2005;111:442-50. [Crossref] [PubMed]
- Narmoneva DA, Oni O, Sieminski AL, et al. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials 2005;26:4837-46. [Crossref] [PubMed]
- Segers VF, Tokunou T, Higgins LJ, et al. Local Delivery of Protease-Resistant Stromal Cell Derived Factor-1 for Stem Cell Recruitment After Myocardial Infarction. Circulation 2007;116:1683-92. [Crossref] [PubMed]
- Jakab K, Norotte C, Damon B, et al. Tissue Engineering by Self-Assembly of Cells Printed into Topologically Defined Structures. Tissue Eng Part A 2008;14:413-21. [Crossref] [PubMed]
- Mironov V, Trusk T, Kasyanov V, et al. Biofabrication: a 21st century manufacturing paradigm. Biofabrication 2009;1. [Crossref] [PubMed]
- Visconti RP, Kasyanov V, Gentile C, et al. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther 2010;10:409-420. [Crossref] [PubMed]
- Takahashi K, Okita K, Nakagawa M, et al. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc 2007;2:3081-9. [Crossref] [PubMed]
- Jung CB, Moretti A, Mederos y Schnitzler M, et al. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med 2012;4:180-91. [Crossref] [PubMed]
- Kawamura M, Miyagawa S, Fukushima S, et al. Enhanced Therapeutic Effects of Human iPS Cell Derived-Cardiomyocyte by Combined Cell-Sheets with Omental Flap Technique in Porcine Ischemic Cardiomyopathy Model. Sci Rep 2017;7:8824. [Crossref] [PubMed]
- Ogasawara T, Okano S, Ichimura H, et al. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci Rep 2017;7:8630. [Crossref] [PubMed]
- Matsuura K, Kodama F, Sugiyama K, et al. Elimination of remaining undifferentiated induced pluripotent stem cells in the process of human cardiac cell sheet fabrication using a methionine-free culture condition. Tissue Eng Part C Methods 2015;21:330-338. [Crossref] [PubMed]
- Chachques JC, Trainini JC, Lago N, et al. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM Trial): Clinical Feasibility Study. Ann Thorac Surg 2008;85:901-8. [Crossref] [PubMed]
- Chachques JC, Trainini JC, Lago N, et al. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant 2007;16:927-34. [Crossref] [PubMed]
- Yoshikawa Y, Miyagawa S, Toda K, et al. Myocardial regenerative therapy using a scaffold-free skeletal-muscle-derived cell sheet in patients with dilated cardiomyopathy even under a left ventricular assist device: a safety and feasibility study. Surg Today 2018;48:200-10. [Crossref] [PubMed]
- Miyagawa S, Domae K, Yoshikawa Y, et al. Phase I Clinical Trial of Autologous Stem Cell–Sheet Transplantation Therapy for Treating Cardiomyopathy. J Am Heart Assoc 2017;6. [Crossref] [PubMed]
- Sawa Y, Yoshikawa Y, Toda K, et al. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ J 2015;79:991-9. [Crossref] [PubMed]
- Frey N, Linke A, Suselbeck T, et al. Intracoronary Delivery of Injectable Bioabsorbable Scaffold (IK-5001) to Treat Left Ventricular Remodeling After ST-Elevation Myocardial Infarction: A First-in-Man Study. Circ Cardiovasc Interv 2014;7:806-812. [Crossref] [PubMed]
- Rao S V., Zeymer U, Douglas PS, et al. Bioabsorbable Intracoronary Matrix for Prevention of Ventricular Remodeling After Myocardial Infarction. J Am Coll Cardiol 2016;68:715-723. [Crossref] [PubMed]
- Lee RJ, Hinson A, Bauernschmitt R, et al. The feasibility and safety of Algisyl-LVRTM as a method of left ventricular augmentation in patients with dilated cardiomyopathy: Initial first in man clinical results. Int J Cardiol 2015;199:18-24. [Crossref] [PubMed]
- Mann DL, Lee RJ, Coats AJS, et al. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail 2016;18:314-25. [Crossref] [PubMed]
- Shum-Tim D, Stock U, Hrkach J, et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg 1999;68:2298-304; discussion 2305. [Crossref] [PubMed]
- Shinoka T, Shum-Tim D, Ma PX, et al. Creation Of Viable Pulmonary Artery Autografts Through Tissue Engineering. J Thorac Cardiovasc Surg 1998;115:536-45. [Crossref] [PubMed]
- Brennan MP, Dardik A, Hibino N, et al. Tissue-engineered Vascular Grafts Demonstrate Evidence of Growth and Development When Implanted in a Juvenile Animal Model. Ann Surg 2008;248:370-7. [PubMed]
- Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 2010;107:4669-74. [Crossref] [PubMed]
- Wissing TB, Bonito V, Bouten CVC, et al. Biomaterial-driven in situ cardiovascular tissue engineering—a multi-disciplinary perspective. NPJ Regen Med 2017;2:18. [Crossref] [PubMed]
- Higgins SP, Solan AK, Niklason LE. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res A 2003;67:295-302. [Crossref] [PubMed]
- Gui L, Zhao L, Spencer RW, et al. Development of novel biodegradable polymer scaffolds for vascular tissue engineering. Tissue Eng Part A 2011;17:1191-200. [Crossref] [PubMed]
- Gui L, Boyle MJ, Kamin YM, et al. Construction of tissue-engineered small-diameter vascular grafts in fibrin scaffolds in 30 days. Tissue Eng Part A 2014;20:1499-507. [Crossref] [PubMed]
- Gui L, Muto A, Chan SA, et al. Development of Decellularized Human Umbilical Arteries as Small-Diameter Vascular Grafts. Tissue Eng Part A 2009;15:2665-76. [Crossref] [PubMed]
- Sugiura T, Matsumura G, Miyamoto S, et al. Tissue-Engineered Vascular Grafts in Children with Congenital Heart Disease: Intermediate Term Follow-up. Semin Thorac Cardiovasc Surg 2018;30:175-9. [Crossref] [PubMed]
- Hopkins RA, Lofland GK, Marshall J, et al. Pulmonary Arterioplasty With Decellularized Allogeneic Patches. Ann Thorac Surg 2014;97:1407-12. [Crossref] [PubMed]
- Lawson JH, Glickman MH, Ilzecki M, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 2016;387:2026-2034. [Crossref] [PubMed]
- Shinoka T, Breuer CK, Tanel RE, et al. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg 1995;60:S513-6. [Crossref] [PubMed]
- Hoerstrup SP, Sodian R, Daebritz S, et al. Functional living trileaflet heart valves grown in vitro. Circulation 2000;102:III44-9. [Crossref] [PubMed]
- Sutherland FWH, Perry TE, Yu Y, et al. From Stem Cells to Viable Autologous Semilunar Heart Valve. Circulation 2005;111:2783-91. [Crossref] [PubMed]
- Gottlieb D, Kunal T, Emani S, et al. In vivo monitoring of function of autologous engineered pulmonary valve. J Thorac Cardiovasc Surg 2010;139:723-31. [Crossref] [PubMed]
- Motta SE, Lintas V, Fioretta ES, et al. Off-the-shelf tissue engineered heart valves for in situ regeneration: current state, challenges and future directions. Expert Rev Med Devices 2018;15:35-45. [Crossref] [PubMed]
- Bertipaglia B, Ortolani F, Petrelli L, et al. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur). Ann Thorac Surg 2003;75:1274-82. [Crossref] [PubMed]
- Lichtenberg A, Tudorache I, Cebotari S, et al. Preclinical Testing of Tissue-Engineered Heart Valves Re-Endothelialized Under Simulated Physiological Conditions. Circulation 2006;114:I-559-65. [Crossref] [PubMed]
- Vincentelli A, Wautot F, Juthier F, et al. In vivo autologous recellularization of a tissue-engineered heart valve: Are bone marrow mesenchymal stem cells the best candidates? J Thorac Cardiovasc Surg 2007;134:424-32. [Crossref] [PubMed]
- Iop L, Renier V, Naso F, et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials 2009;30:4104-16. [Crossref] [PubMed]
- Iop L, Bonetti A, Naso F, et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS One 2014;9. [Crossref] [PubMed]
- Gallo M, Bonetti A, Poser H, et al. Decellularized aortic conduits: could their cryopreservation affect post-implantation outcomes? A morpho-functional study on porcine homografts. Heart Vessels 2016;31:1862-73. [Crossref] [PubMed]
- Reuven EM, Leviatan Ben-Arye S, Marshanski T, et al. Characterization of immunogenic Neu5Gc in bioprosthetic heart valves. Xenotransplantation 2016;23:381-92. [Crossref] [PubMed]
- Iop L, Paolin A, Aguiari P, et al. Decellularized Cryopreserved Allografts as Off-the-Shelf Allogeneic Alternative for Heart Valve Replacement: In Vitro Assessment Before Clinical Translation. J Cardiovasc Transl Res 2017;10:93-103. [Crossref] [PubMed]
- Xplore2 clinical trial. Available online: https://xplore2trial.com/
- Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 2003;23:1002-6; discussion 1006. [Crossref] [PubMed]
- Spark JI, Yeluri S, Derham C, et al. Incomplete cellular depopulation may explain the high failure rate of bovine ureteric grafts. Br J Surg 2008;95:582-5. [Crossref] [PubMed]
- Sarikouch S, Horke A, Tudorache I, et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 2016;50:281-90. [Crossref] [PubMed]


