Single-port video-assisted thoracic surgery (VATS)—advanced procedures & update
Introduction
Uniportal thoracoscopy has evolved rapidly during a short period of time, with simple procedures in its beginnings to uniportal lobectomies for cancer (1), and more recently with highly complex surgeries such as tracheal resection and reconstruction (TRR) (2), bronchoplastic procedures (3), esophagectomies (4), and resection of advanced lung neoplasms together with chest wall (5).
It is already known that the uniportal video-assisted thoracic surgery (VATS) approach may have potential advantages, including less postoperative pain, faster recovery and a better cosmetic outcome, preserving a good hilar exposure and the ergonomics of the procedure with a small incision (6-8).
The first report of a uniportal VATS procedure was in the year 2000, with a uniportal sympathectomy (9). It took 10 years for the first reported uniportal VATS lobectomy by Dr. González-Rivas, proving that a major pulmonary resection is achievable (1). After that, publications appeared with more complex uniportal procedures, such as segmentectomy and pneumonectomy (10,11).
The most complex and challenging procedures for thoracic surgeons are bronchoplastic procedures, carinal and tracheal resections, and lobectomies with chest wall en bloc resections.
Improvements in technology
The rapid growth and adoption of the uniportal approach has pushed the development of technology in almost every field where VATS surgery is applicable. High definition cameras, new generation thoracoscopes with a range of vision from 0° up to a 120° gives an excellent exposure of the surgical field without torqueing of the scope. Also, 3D imaging technology has come in handy, adding depth perception and facilitating faster and more accurate grasping and suturing during surgery (3,12).
The robotic arm for the thoracoscope also allow performing “unisurgeon” uniportal lung resections, providing more space for the surgeon to work comfortably, while the assistant is nearby in case the first surgeon needs any help during the surgery (Figure 1). Improvements in visualization devices are still to come with the wireless thoracoscope development in the near future, as well as the magnetic anchorage guidance system (MAGS) that uses magnets to hold the camera to the internal side of thoracic wall, providing a large view angle and a wide range of movement inside the thoracic cavity, leaving the incision free for instrumentation (13).
Staplers have also become more ergonomic and adapted to uniportal VATS, with curved tip stapler technology (Covidien, Mansfield, MA, USA) and narrower staplers (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) which considerably facilitate the passage of the stapler through vascular structures, specially convenient for upper lobe veins, as the tip reduces the chances of damaging to the pulmonary artery (PA) (13).
Retraction instruments using magnets have also been developed to hold the lung grasper to the thoracic wall and retract the lung in any direction necessary, allowing more space in the incision for dissection instruments. Technology will continue to improve in every aspect of the VATS approach, giving surgeons more resources to perform more procedures with higher levels of complexity without compromising comfort permitting a widespread of the technique.
Bronchoplastic procedures
Lung cancer is a complex disease that requires a multimodality approach. In the surgical field, a trend to a less invasive procedure has become the ultimate goal, preserving functional lung parenchyma as much as possible while maintaining oncological safety. As a result of this trend and given the improvement in VATS technology, complex procedures, such as bronchoplastic and bronchovascular reconstruction, is being now performed by conventional VATS (14). In recent years, uniportal VATS has emerged as a less invasive procedure that can be used by experienced surgeons in complex lung resections with sleeve reconstructions (3,15).
In these procedures, the same principles as open surgery is applied, keeping a direct view with the eyes above the surgeon’s hands (16). To facilitate the procedure, there are a number of devices the surgeon can use to make the surgery more comfortable. A plastic wound retractor will prevent the interference of fatty tissue with the thread during suturing and using an energy device during dissection will provide more hemostasis and a cleaner field. Proximal and distal articulation instruments are mandatory for single port reconstructive procedures. The patient’s position is also very important, rotating the table 45° degrees anteriorly, in direction to the surgeon will expose the anastomosis site providing a better view, especially during the suturing of the posterior bronchial wall (3).
Bronchoplastic procedures can be divided into three types, according to the resection type:
- Simple bronchoplasty: it is performed when the tumor is located in the bronchial base and the bronchus is resected using a scalpel and scissors. To suture the defect, a continuous suture of an absorbable monofilament is preferred, but interrupted sutures are an available option for surgeons who prefer this technique;
- Wedge bronchoplasty: a wedge incision is made deeper in the main bronchus and is repaired with a transverse closure, unless the wedge is too large and approximation is unsatisfactory. In this case a lateral closure can be performed;
- Sleeve bronchoplasty: in this case, a segment of the bronchus is resected and an end to end anastomosis must be performed to allow airway reconstruction. In the early experience, the anastomosis was performed with a combination of interrupted and continuous sutures, performing the membranous wall with continuous sutures and the anterior bronchial wall with interrupted sutures (17). Nowadays, a continuous suture for the 360° of the anastomosis including membranous and cartilaginous portion seems more suitable, reduces surgical time and facilitates the procedure for the surgeon (18) (Figure 2).
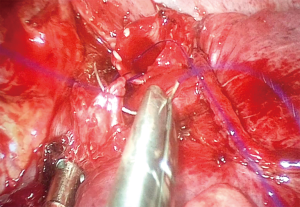
Skilled surgeons are able to perform sleeve lobectomies, both in the right and left lung lobes, but the right upper lobe (RUL) sleeve lobectomy is technically less demanding, as the alignment of the right main bronchus and the intermediate bronchus facilitate the anastomosis, especially if the incision is made in the anterior axillary line, providing a more direct approach. The paratracheal lymph node dissection should be performed after completion of the anastomosis to avoid retraction of the right main bronchus behind the PA or the azygos vein. Dissection and releasing the pulmonary ligament allows a free tension anastomosis and should always be performed (18).
Left side sleeve lobectomies are the most technically challenging cases, since the larger size of the PA and the retraction of the left main bronchus (LMB) below it result in a very difficult anastomosis. The presence of the aortic arch and the absence of the intermediate bronchus also adds complexity to the procedure. For example, in the left upper lobe (LUL) sleeve lobectomy, division of the bronchus occurs just proximal and distal to the base of the LUL, so when the surgeon divides the distal end care must be taken to avoid injury of the superior segmental bronchus. It is advisable to do the subcarinal and aortopulmonary window lymph node dissection before the anastomosis, to avoid trauma and traction after completion (18).
The left lower sleeve lobectomy is the most difficult procedure due to interference of the anastomosis field by the PA, atrium and LUL vein. Reconstruction is more complicated, since the LUL lobe bronchus is re-implanted unto the LMB in an anterior view position, and the LMB is usually deeply retracted below the PA (18).
Usually, in all sleeve lobectomies, the bronchus is incised and resected in the same manner; first an incision is made in the anterior wall with a long scalpel and then, the circumferential resection is completed with distal articulation scissors. Experience has shown that the most feasible and less time-consuming anastomosis is carried out with a running suture for both the posterior and anterior bronchial wall, and should be always performed using the same technique, since it will allow the surgeon to improve their skill with each case, reducing the time spent performing the anastomosis and the technical challenges inherent to these complex cases (18,19).
Lung sparing bronchial sleeve resections are usually more demanding for the surgeon, as the field of exposure is diminished due to the presence of the entire lung structures, because they are preserved for these cases. Dissection also involves a higher degree of complexity, since the bronchial segment needs to be exposed and isolated for reconstruction, which requires meticulous dissection, preservation, and careful retraction of overlying structures. The anastomosis is usually carried out in the same manner as for sleeve lobectomies. Lung sparing procedures should be attempted for low grade malignant tumors or benign conditions anatomically located in a site that allows for this procedure to be completed successfully, such as the intermediate bronchus or the right main bronchus (19).
Vascular reconstruction
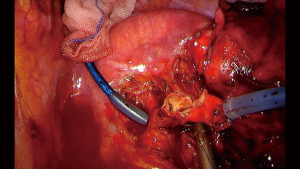
When the procedure performed involves some degree of vascular reconstruction, it is always advisable to obtain control of the PA and the hilum at the beginning of the procedure. Good exposure is mandatory and instrumentation during the reconstruction should be as comfortable as possible. Keeping this in mind, clamping of the PA can be done with a thoracoscopic clamp, but using a tourniquet system with a thread allows control of the PA without interference the instrumentation through the incision, since it can be placed safely inside the thoracic cavity (Figure 3). For distal clamping the same principle can be used or using a bulldog clamp in the distal artery or inferior pulmonary vein instead of the interlobar artery will facilitate less interference with the reconstruction (18). The extent of the reconstruction will depend on the extent of the compromise of the PA, since partial resection and primary reconstruction can be attempted if less than 1/3 of the PA is compromised. For more extensive compromise an end to end sleeve vascular reconstruction is recommended (18).
Once the vascular anastomosis is going to be performed, heparinization with 5,000 IU intravenous heparin should be done before the clamping of the PA. In case of double sleeve lobectomies, the bronchial anastomosis should be performed first to avoid traction and trauma to the vascular anastomosis. A running 360-degree suture with a non-absorbable monofilament (polypropylene 5-0, one threat with two needles) is completed from back to front, starting with the posterior wall and finishing in the anterior wall. It is advisable to release the distal tourniquet or clamp before the knot is tied, to fill the artery with blood flow, to allow flushing out any air. After tying the knot, a progressive release of the proximal tourniquet or clamp will allow visualization of any bleeding between the sutures that would requires another suture before completely removing of the clamp (18).
Lobectomies with chest wall resection
Indications for lung cancer surgery have been changing over time. In its origins, only initial stages were considered for a VATS procedure; and since 2010, with the first reported uniportal lobectomy (1), the development of technologies supporting VATS techniques and the ability to perform complex procedures by experienced surgeons, it is now feasible to perform difficult surgeries for advanced lung cancer with chest wall invasion with a minimally invasive technique (5).
Reports exist of primary chest wall tumors resections using VATS. Rib resection is typically performed with a large skin incision and soft tissue dissection, but it is possible with a thoracoscopic approach from the inner side of the chest wall instead of the outside, to decrease pain and improve recovery (20). Several reports use a multiportal approach (21). Huang and colleagues published a case report of a single port rib resection VATS without the need for chest wall reconstruction (22). Ribs can be transected with Gigli saws, endoscopic shears, or high-speed drills. For other primary chest wall masses, selective reconstruction may be required, using synthetic materials, which also can be done by VATS (20). Experience with thoracoscopic resection of chest masses has served as a precedent for excision of locally invasive lung tumors with compromised chest wall.
The decision to perform a VATS approach in a resectable chest wall invasion lung cancer arises from the perception that perturbing the rib cage with a smaller incision, avoiding rib spreading and the incision of other chest wall tissue, will have a better outcome, with less postoperative pain, with the same oncologic results as with an open surgery (23,24). Afterwards, a large number of thoracoscopic lobectomies with rib resection were performed using 3 to 4 incisions (25).
The first report of a multi-port VATS with en bloc resection was done by Widmann et al. in 2000. They performed a LUL wedge resection with two ribs in a patient with a T3 post neoadjuvant radiotherapy lung adenocarcinoma (26). Bayarri and colleagues reported uniportal VATS for exploring pleural cavity with an anterior incision for intraoperative staging, and then, they delineated the boundaries of chest wall involvement with hypodermic needles to help them place the incision at the exact place of chest wall invasion, thus preventing a more extensive thoracotomy (24).
A series of 105 patients underwent en bloc lobectomies and chest wall resections, with 93 open thoracotomies and 12 patients via hybrid thoracoscopic multiport approach. Postoperative outcomes were similar between these two groups, with a shorter hospitalization length of stay in the VATS group. They used a limited counter incision without rib spreading for the chest wall resection (25).
It is important to take in consideration an appropriate patient selection for the success of this kind of hybrid thoracoscopic procedure: the size of the tumor, the presence of hilar adenopathy, the use of prior radiation therapy, and the location of the chest wall involvement. Tumors that require the resection of the first rib are particularly difficult to manage by VATS, due to the dissection near the thoracic outlet, as well as limited visualization at the apex of the thorax (27).
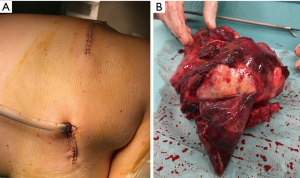
It was until 2013 that González-Rivas did the first uniportal VATS lobectomy with en bloc chest wall resection in a patient with an adenocarcinoma of RUL with posterior chest wall invasion. They performed the procedure with a single anterior incision for a uniportal upper lobectomy, and a posterior approach for the chest wall resection (total of two incisions). With this technique, they avoided injury to as few intercostal spaces as possible, while preserving the oncologic procedure. The posterior or lateral incision gives confidence to surgeon for a complete oncologic resection, taking control from outside and inside, and achieving good oncological margins. This hybrid technique is very useful in case of Pancoast tumors or big tumors in order to avoid large thoracotomies (Figure 4). Also, in cases where a chest wall reconstruction is necessary, the posterior incision allows easy placement of a prosthesis (5). The same author performed similar procedures later using single port VATS with chest wall excision and reconstruction with mesh (28) and pure uniportal procedures with chest wall resection through the same incision (29).
Recently, Jaus and colleagues published a series of three cases using hybrid approach with assisted video thoracoscopic lobectomy and a chest wall en bloc resection, with an alternative method for estimating thoracic wall resection which uses assisted video surgery and hypodermic needles (minimally invasive posterior approach). They performed an anterior utility incision for the upper right lobectomy and, under direct visualization, marked a 2-cm margin of the chest wall invasion with hypodermic needles. Then they did an en bloc resection of the chest wall with three ribs with a second posterior incision without rib spreading. The authors reported a good postoperative outcome without major mortality (30).
This minimally invasive approach permits operative times to be gradually reduced, while gaining experience. The patient presents less postoperative pain with a faster recovery and a reduced need of prosthetic reconstruction (20,23,28).
Carinal and tracheal resection
Carinal and tracheal surgery is without a doubt one of the most complex and challenging procedures for thoracic surgeons (31). Even in high volume centers, carinal surgery through open thoracotomy carries high morbidity (39–50%) and mortality (2.2–7.1%) (32), and for tracheal resection, a mortality of 3–11% (33) with 15% of anastomotic failure (34).
Coordination between the surgeon and the anesthesiologist is imperative and a preoperative discussion among the team members involved is necessary in case trans-operative complications occur (13,34).
Carinal reconstructive surgery has been performed by conventional VATS (32,35). Li and colleagues, in a series of 12 cases, demonstrated that VATS resection and reconstruction of the carina or trachea is safe and feasible, and these procedures can be safely performed using a multiportal approach in selected patients (36). Three or more incisions were usually used during a thoracoscopic tracheal and carinal resection and reconstruction (34,36,37).
The two main concerns for this procedure are reduction of anastomotic tension and correct choice of the type of reconstruction, regardless of the surgical approach (open or VATS) (38). Before the dissection of the carina, it is advisable to release the inferior pulmonary ligament to avoid excessive tension to the anastomosis and to maximize exposure by transecting the azygous vein. Lymph node dissection can be done after resection, but before reconstruction, avoiding excessive devascularization of the airway. If a tension-free carinal reconstruction anastomosis cannot be successfully performed, then the surgeon should perform a carinal pneumonectomy with an end to end anastomosis of the trachea to the LMB (18).
The choice for carinal reconstruction will depend on the extent of the resection and usually involves a “double barrel” technique, forming a neocarina and then attaching it to the trachea for purely limited resections of the carina. The suture starts with the left side of the trachea and LMB, then proceeds with membranous wall of the trachea and LMB, then the neocarina and finishes with the right side of the trachea, right main bronchus and anterior wall of the LMB. When a right upper lobectomy is necessary careful reimplantation of the intermedius or right lower bronchus to the trachea or LMB is carried out to avoid ischemia, necrosis, or stenosis (18).
To date, only González-Rivas and the Tyumen team have reported uniportal carinal resections (39), but as experience grows and technology is developed, more team will be able to carry out these complex procedures through a uniportal approach.
Traditionally, the most commonly employed approach to the mediastinal trachea is a right lateral thoracotomy, taking into consideration the alternative of a median sternotomy with dissection between the superior vena cava and the aorta (34,40). The minimally invasive approach may have the advantage of providing a better and magnified operation field, as opposed to an open thoracotomy, where the operation field for the upper-to-middle portion of the mediastinal trachea may be limited, as it is located near the apex of pleural cupula (40).
The first reported multiport tracheal resection and carinal reconstruction using a cross-field ventilation in a patient with tracheal tumor was done by He and colleagues, with a patient presenting an adenoid cystic carcinoma of the distal trachea, extending along the right main bronchus with carinal invasion, demonstrating that VATS tracheal resection and carinal reconstruction is a feasible option for patients with tracheal tumor with carina involvement (41).
The first report of uniportal tracheal and carinal resections was reported by González-Rivas and colleagues in 2015. In this publication the technique of tracheal and complex carinal reconstructions is described in detail by using high frequency ventilation jet (42).
Since this initial publication a very few articles have been described in the literature. Liu et al. published in 2017 a case report of a tracheal resection by using an intrafield endotracheal tube through the single incision. The patient had a lower third primary tracheal adenoid cystic carcinoma, and underwent a uniportal VATS approach through a single 3-cm incision in the right fourth intercostal space, using a 3/0 polypropylene continuous suture and maintaining ventilation with an intra-surgical field endotracheal tube, with a good postoperative outcome (43). Hung and colleagues reported a patient with tracheal squamous cell carcinoma. They used single-lumen endotracheal intubation with a long nasal tube, which was then inserted into the LMB for one-lung ventilation under bronchoscopic guidance. They did a 4-cm single incision and performed the tracheal anastomosis with two 3-0 knotless continuous suture, reinforced with a fibrin sealant, maintaining the endotracheal tube in situ, without postoperative complications (44). Single-port thoracoscopic tracheal resection appeared to be feasible and safe, as shown in previously reported single cases. However, with such few cases reported, more evidence is necessary to take a the most appropriate decisions (43,44).
For tracheal anastomosis, continuous and interrupted suturing are both used for the membranous and cartilaginous portions of trachea. The advantages of this hybrid approach are that the continuous sutures avoid tangling the ends and shortening anastomosis time, and the interrupted sutures prevent anastomosis leak and stricture often caused by continuous suturing. On the other hand, placing interrupted sutures using a VATS approach can be more complex and time-consuming than using a running suture. The use of continuous running sutures provides a better operative view and has been proven safe and effective in VATS (45).
The lack of sufficient experience in tracheal and carinal surgery is a problem to solve. The surgeon must be sufficiently experienced in VATS lobectomy and in open extended resections, such as sleeve lobectomy and carinal resection, before planning thoracoscopic tracheal surgery. Also, the anesthesiologist plays a key role in airway management, which is a critical issue in tracheal surgery. VATS procedures may be preferably indicated for benign or low-grade malignant tumors with a shorter extension (40).
To maintain lung ventilation during carinal and tracheal resection the surgeon has different options, such as an intra-surgical field bronchial intubation (46) or through high frequency jet ventilation (HFJV) (47).
An intra-surgical field intubation consists of a sterile circuit that is passed through the operative field and prepared to directly ventilate a single lung. The additional cross-field ventilation is a successful option in different TRR and carinoplasties and is, therefore, used in the vast majority of procedures. One of the disadvantages of this technique is that the cross-field tube can sometimes obstruct the view of the reconstruction site, thus periodical retraction of the tube may be necessary when performing anastomosis in order to improve exposure (38). Therefore, for VATS approach is also advisable to insert the cross-field tube through a separate thoracic incision, although it can be used in the same incision when used in a uniportal approach (43), and it has the advantage that the cuffed tube prevents the spilling of blood into the distal airway, which could lead to obstruction of the lung that actually needs to be ventilated (38).
HFJV consists of the application of gas portions under high pressure through a catheter into the airway which is open to ambient air (48). This HFJV catheter has a small diameter and it can be introduced through the endotracheal tube. Therefore, it solves the problem of obstruction of view for the anastomotic site in a TRR, and it doesn’t require another incision for a cross field tube. A drawback is the moderate ventilation of the lung on the operative site which may affect exposure, especially when using a minimally invasive approach (38). It is important to take in consideration that some difficulties can be encountered in maintaining oxygenation and CO2 elimination in patients with morbid obesity, stiff thorax and advanced forms of restrictive or obstructive pneumopathy (48).
The extracorporeal membrane oxygenation (ECMO) and the extracorporeal life support is useful in the care of patients with near total occlusion of the airways to preserve the respiratory functions during a complex surgery. The ECMO provides a tubeless operation field in a TRR, so veno-venous (V-V) ECMO could be useful for uniportal VATS and airway surgery. It is also a tool for management of adequate oxygenation in the context of an emergency airway surgery, when it is impossible to ventilate the patient (45,49). The disadvantages of using ECMO involve a more invasive approach that requires vascular cannulation and the amount of blood loss in the circuit once the patient is decannulated.
Non-intubated advanced procedures
Advanced thoracic procedures under spontaneous breathing without endotracheal intubation can avoid mechanical ventilation complications, like barotrauma, atelectrauma, volutrauma, and biotrauma, and it can avoid muscle relaxation, maintaining the respiratory functional capacity and avoiding atelectasis in the dependent lung (50), giving the patient an even lesser minimally invasive approach.
The first report of a non-intubated uniportal VATS major resection was in 2014 by González-Rivas and colleagues, when they performed a middle lobectomy with lymphadenectomy in a patient in spontaneous ventilation (51). Since then, it has been used in different procedures with good results.
The use of spontaneous ventilation without endotracheal tube gives the surgeons an advantage in complex carinal and tracheal VATS procedures because it allows an unobstructed view of the tracheal and carinal anastomosis, hence, the surgeon can do a faster anastomosis with a reduction in operative time (52) (Figure 5). The benefits of this technique in tracheal resections were shown by Jiang and colleagues with a series of patients who underwent a TRR and carinal resection, most of the procedures performed with a tri-port approach, where the authors reported a shorter operative time and a potentially faster recovery than the conventional procedure with an endotracheal tube (31,52).
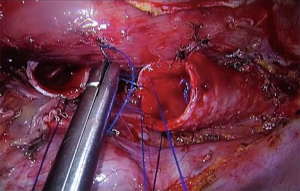
It is feasible that non-intubated anesthesia can be used also in complex uniportal VATS. There are reports of single port thoracoscopic non-intubated bronchial sleeve resections and in carinal reconstruction with excellent outcomes (53,54).
Conclusions
The experience acquired with minimally invasive techniques such as the uniportal VATS approach, allows expert surgeons to perform advanced procedures including VATS bronchovascular and carinal sleeve resections or tumors involving the chest wall, with good postoperative outcomes.
High volume experience is important for the surgical outcome, so these advanced procedures must be done by surgeons with vast experience with the single port technique, so they can have the ability to confidently and safely perform various types of complicated VATS procedures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact CardioVasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Liu Z, Yang R, Shao F. Uniportal Video-Assisted Thoracoscopic Tracheal Resection. Ann Thorac Oncol Res 2017;1:1004.
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Dmitrii S, Pavel K. Uniportal Video-Assisted Thoracic Surgery Esophagectomy. Thorac Surg Clin 2017;27:407-15. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations 2013;8:70-2. [PubMed]
- Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. [Crossref] [PubMed]
- Halezeroğlu S. Advantages and disadvantages of single incision VATS in major anatomical resection for lung cancer. J Vis Surg 2017;3:115. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Nesher N, Galili R, Sharony R, et al. Videothorascopic sympathectomy (VATS) for palmar hyperhidriosis:summary of a clinical trial and surgical results. Harefuah 2000;138:913-6, 1008. [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Video: single incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc 2012;26:2078-9. [Crossref] [PubMed]
- Ismail M, Swierzy M, Nachira D, et al. Uniportal video-assisted thoracic surgery for major lung resections: pitfalls, tips and tricks. J Thorac Dis 2017;9:885-97. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Calvin NG. Advances in uniportal video-assisted thoracoscopic surgery: pushing the envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Terzi A. Moving beyond the boundary: the emerging role of video-assisted thoracic surgery for bronchoplastic resections. J Thorac Dis 2014;6:1170-2. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Davoli F, Bertolaccini L, Pardolesi A, et al. Video-assisted thoracoscopic surgery bronchial sleeve lobectomy. J Vis Surg 2017;3:41. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Sekhniaidze D, et al. Uniportal video-assisted thoracoscopic bronchoplastic and carinal sleeve procedures. J Thorac Dis 2016;8:S210-22. [PubMed]
- Sardenberg RA, Gonzalez-Rivas D. Uniportal video-assisted thoracic surgery right main sparing lung bronchial sleeve resection: live surgery in Sao Paolo. J Vis Surg 2017;3:166. [Crossref] [PubMed]
- Demmy TL, Yendamuri S, Hennon MW, et al. Thoracoscopic maneuvers for chest wall resection and reconstruction. J Thorac Cardiovasc Surg 2012;144:S52-7. [Crossref] [PubMed]
- Hennon MW, Demmy TL. Thoracoscopic resection and re-resection of an anterior chest wall chondrosarcoma. Innovations 2012;7:445-7. [PubMed]
- Huang CL, Cheng CY, Lin CH, et al. Single-port thoracoscopic rib resection: a case report. J Cardiothorac Surg 2014;9:49. [Crossref] [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 1934-5.
- Bayarri CI, de Guevara AC, Martin-Ucar AE. Initial single-port thoracoscopy to reduce surgical trauma during open en bloc chest wall and pulmonary resection for locally invasive cancer. Interact Cardiovasc Thorac Surg 2013;17:32-5. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Widmann MD, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgery resection of chest wall en bloc for lung carcinoma. Ann Thorac Surg 2000;70:2138-40. [Crossref] [PubMed]
- Giaccone A, Solli P, Pardolesi A, et al. Video-assisted thoracoscopic surgery en bloc chest wall resection. J Vis Surg 2017;3:73. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Lower lobectomy with chest wall resection. Asvide 2014;1:347. Available online: http://www.asvide.com/articles/360
- Gonzalez-Rivas D, Xie B, Yang Y, et al. Uniportal video-assisted thoracoscopic lobectomy with en bloc chest wall resection. J Vis Surg 2015;1:7. [PubMed]
- Jaus MO, Forcione A, Gonfiotti A, et al. Hybrid treatment of T3 chest wall lung cancer lobectomy. J Vis Surg 2018;4:32. [Crossref] [PubMed]
- Li J, Liu H, Liu J, et al. Challenges in complex video-assisted thoracoscopic surgery and spontaneous respiration video-assisted thoracoscopic surgery procedures. J Vis Surg 2017;3:31. [Crossref] [PubMed]
- Nakanishi R, Oda R, Sakane T, et al. Video-assisted thoracoscopic surgery (VATS) for central airway tumors: VATS carinal resection and reconstruction. Video-assist Thorac Surg 2018;3:7. [Crossref]
- Hobai IA, Chhangani SV, Alfille PH. Anesthesia for tracheal resection and reconstruction. Anesthesiol Clin 2012;30:709-30. [Crossref] [PubMed]
- Blasberg JD, Wright CD. Surgical considerations in tracheal and carinal resection. Semin Cardiothorac Vasc Anesth 2012;16:190-5. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Muranaka K, et al. Surgical Techniques: Thoracoscopic carinal resection and reconstruction in a patient with mucoepidermoid carcinoma. J Thorac Cardiovasc Surg 2013;145:1134-5. [Crossref] [PubMed]
- Li J, Wang W, Jiang L, et al. Video-assisted thoracic surgery resection and reconstruction of carina and trachea for malignant or benign disease in 12 patients: Three centers’ experience in China. Ann Thorac Surg 2016;102:295-303. [Crossref] [PubMed]
- Li S, Liu J, He J, et al. Video-assisted thoracoscopic surgery resection and reconstruction of thoracic trachea in the management of a tracheal neoplasm. J Thorac Dis 2016;8:600-7. [Crossref] [PubMed]
- Kocher GJ, Dorn P. Minimally invasive resection and reconstruction of the intrathoracic trachea and carina. J Thorac Dis 2017;9:4319-22. [Crossref] [PubMed]
- Lyscov A, Obukhova T, Ryabova V, et al. Double-sleeve and carinal resections using the uniportal VATS technique: a single centre experience. J Thorac Dis 2016;8 Suppl 3:S235-41. [PubMed]
- Imanishi N, Tanaka F. Thoracoscopic tracheal resection and reconstruction: video-assisted thoracoscopic surgery as a “tool” toward minimally invasive surgery. J Thorac Dis 2017;9:2895-7. [Crossref] [PubMed]
- He J, Wang W, Li J, et al. Video-assisted thoracoscopic surgery tracheal resection and carinal reconstruction for tracheal adenoid cystic carcinoma. J Thorac Dis 2016;8:198-203. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i6-16. [PubMed]
- Liu Z, Yang R, Shao F. Anastomosis using complete continuous suture in uniportal video-assisted thoracoscopic bronchial sleeve lobectomy. Minim Invasive Surg Oncol 2017;1:31-42.
- Hung WH, Chen HC, Huang CL, et al. Thoracoscopic Tracheal Resection and Reconstruction with Single-Incision Method. Ann Thorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Ujiie H, Yasufuku K. New era of “resection of the carina and lower trachea”. J Thorac Dis 2017;9:4932-6. [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. Thorac Surg Clin 2014;24:77-83. [Crossref] [PubMed]
- Watanabe Y, Murakami S, Iwa T, et al. The clinical value of high-frequency jet ventilation in major airway reconstructive surgery. Scand J Thorac Cardiovasc Surg 1988;22:227-33. [Crossref] [PubMed]
- Biro P. Jet ventilation for surgical interventions in the upper airway. Anesthesiol Clin 2010;28:397-409. [Crossref] [PubMed]
- Hoetzenecker K, Klepetko W, Keshavjee S, et al. Extracorporeal support in airway surgery. J Thorac Dis 2017;9:2108-17. [Crossref] [PubMed]
- Gonzalez-Rivas D, Aymerich H, Bonome C, et al. From open operations to nonintubated uniportal video-assisted thoracoscopic lobectomy: Minimizing the trauma to the patient. Ann Thorac Surg 2015;100:2003-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Jiang L, Liu J, Gonzalez-Rivas D, et al. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Shao W, Phan K, Guo X, et al. Non-intubated complete thoracoscopic bronchial sleeve resection for central lung cancer. J Thorac Dis 2014;6:1485-8. [PubMed]
- Peng G, Cui F, Ang KL, et al. Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. [Crossref] [PubMed]