Video-assisted thoracoscopic surgery lobectomy with chest wall resection
Introduction
As recently as 1947, Coleman and colleagues reported the first surgical treatment of lung cancer invading the chest wall (1). Although for some time, chest wall invasion was considered a contraindication for upfront resection (2), nowadays, despite some controversies, non-small cell lung cancer (NSCLC) invading the parietal pleura and the chest wall is primarily a surgical disease (3). Lung cancers that infiltrate the parietal pleura, subpleural soft tissue or bony structures of the chest wall by direct contact are T3 tumors according to the tumor, node and metastasis (TNM) lung cancer staging system (4), and occur in only 5–8% of reported cases (5). In the absence of lymph node metastasis, 5-year survival is 40–50% (2,3,6-16). Superior sulcus tumors or Pancoast tumors occur in 1% of patients and will not be discussed in this article.
Minimally invasive approaches to lobectomy in early-stage lung cancer have been demonstrated safe and effective, with equivalent oncologic outcomes to open thoracotomy and lower overall complication and mortality rates (17). Despite controversies regarding feasibility and completeness of resection, thoracic surgeons who work in high-volume centers keep pushing the limits to perform complex procedures, also known as “extended resections”, through minimally invasive surgery. Published series and case reports have confirmed the safety and efficacy of video-assisted thoracoscopic surgery (VATS) in highly complex surgical cases, such as lobectomy with chest wall resection of locally advanced lung cancer (18-21). In this article, we will present the clinical indications of locally advanced, T3 NSCLC that infiltrates the chest wall and the technical aspects of accomplishing a safe and oncologically sound extended resection through VATS.
Clinical presentation
As a rule, patients with lung cancer with chest wall invasion will present with thoracic pain, although the absence of pain does not exclude the possibility of chest wall infiltration (22), and only 50% of the patients with thoracic pain will, in fact, present chest wall invasion (10). Localized pain in association with a lung cancer that has intimate contact with the chest wall is a highly specific sign of chest wall infiltration. The pain is described as severe, continuous, and burning-like and does not respond to conventional analgesics (10). Paulson described it as ‘excruciating’ pain (10). As with other lung cancers, patients with chest wall invasion may also present with cough, hemoptysis, dyspnea, or no symptoms at all (23,24).
Lung cancer staging
According to international guidelines, patients with suspected or proven lung cancer should undergo chest computed tomography (CT), positron emission tomography (PET), and brain imaging as part of the clinical oncological staging (25,26). Because lung cancer with chest wall invasion is classified as a locally advanced tumor, all patients should undergo invasive mediastinal staging, either by endobronchial ultrasonography (EBUS) or mediastinoscopy, to exclude mediastinal lymph node metastasis (17). Resectability is defined by the presence or absence of involved mediastinal lymph nodes or distant metastases (25,26).
It is critical to properly grade chest wall invasion preoperatively, because there is direct correlation between the level of infiltration and prognosis, as demonstrated by the work of Magdeleinat, Facciolo, and their colleagues (6,13). The depth of invasion is also an unfavorable prognostic factor (6,13). One theory is that intercostal muscle or rib invasion promotes distant metastasis because these structures are richly supplied with blood (23). Ultrasound, CT, and magnetic resonance imaging (MRI) are the standard imaging tests available to evaluate chest wall infiltration. Ultrasound is probably used the least due to the rare indications and operator-dependent limitations (27). CT scan has a good sensitivity, up to 50–81%, to correctly define the level of chest wall invasion (28,29). It is important to understand that a simple contact between the tumor and the chest wall or neighboring structures does not necessarily imply cancer invasion (30,31). MRI may better define the level of infiltration, because it increases the chances of precise chest wall evaluation; respiratory dynamic MRI demonstrated a sensitivity of 100% and specificity of 82.9% (32).
Invasive mediastinal staging with systematic sampling is a critical part of the preoperative evaluation, because the presence of N2 disease in combination with a T3 lung cancer due to chest wall invasion significantly affects overall survival (2,33). Facciolo and colleagues reported a 5-year overall survival of 18% for patients with T3N2 disease as compared with 61% 5-year survival in the absence of lymph node metastasis (T3N0) (13). In high-volume lung cancer centers, EBUS is the preferred modality to preoperatively investigate the mediastinum due to its efficacy, minimal invasiveness, and cost-effectiveness (33,34). If the CT scan or PET-CT is suspicious for N2 disease and the EBUS is negative, the added value of mediastinoscopy is well established (28). In fact, because N2 disease imposes a low survival rate, it is advisable to start the surgical resection with lymphadenectomy to validate the node-negative clinical staging. Even in clinical N0 patients, frozen sections of the mediastinal lymph nodes should be examined, and if N2 disease is found, resection should probably be aborted.
Surgical resection
It is universally accepted that nodal status and completeness of resection of the chest wall have significant prognostic value in predicting disease-free survival and overall survival of lung cancer patients with chest wall involvement. For example, a series by Voltolini and colleagues in 2006 demonstrated through a multivariate analysis that node-negative patients with negative margins of resection have superior long-term survival (35). Likewise, others have demonstrated similar results (2,12,36).
It is simple to validate the nodal status determined preoperatively during surgery, because the correlation of frozen-section analyses and definitive pathology is very precise. In contrast, the evaluation of resection margins during surgery has limitations because it is based on the combination of microscopic and macroscopic analysis of the specimen. In chest wall resection, a 1-cm margin in all directions is generally accepted; however, some advocate for a margin of 1 intact rib above and below the tumor and a 3–4 cm lateral margin (13). Frozen sections are not performed on resected bone samples (37). R1 or R2 resection remain the most reproducible independent predictor of poor survival in all published series of lung cancer with chest wall infiltration (22). Because complete resection is the final objective, the surgeon and the pathologist should work together to achieve an R0 result.
There is some controversy regarding the best type of surgical resection for T3 tumors infiltrating the chest wall. The extent of surgical resection depends on the depth of invasion and the tumor location. For tumors limited to the parietal pleural, extrapleural resection keeping the chest wall intact is sufficient to achieve complete resection with long-term survival (2,38). In cases of isolated parietal pleura invasion, chest wall resection does not convey any survival advantage (2). When soft tissue and ribs are involved, extrapleural dissection should be avoided and upfront resection of the chest wall should be performed.
En bloc lung and chest wall resection is associated with a morbidity rate above 20% and mortality ranging from 3.8% to 7%, both are higher than expected rates for VATS lobectomy alone (39,40). In fact, a recent large database analysis demonstrated that patients who underwent lobectomy with en bloc chest wall resection experienced 90-day mortality comparable with that of patients who underwent pneumonectomy (11). Pulmonary complications were the most common cause of death in patients who underwent chest wall resection and reconstruction (41). We believe that less invasive procedures may help decrease early complications and, therefore, provide better short-term results (38).
Adjuvant and neoadjuvant therapy
The role of adjuvant therapy for T3N0 tumors that invade the chest wall is controversial (42). Based on most published data, complete resection should be the primary goal for proper oncological management of NSCLC invading the chest wall. In patients with node-negative tumors ≥4 cm in diameter, adjuvant chemotherapy might be offered based on a subset analysis of the Cancer and Leukemia Group B (CALGB) 9633 study, which found a statistically significant survival advantage of adjuvant chemotherapy after complete tumor resection of tumors ≥4 cm (43). If the surgical margins are positive, however, the patient should undergo further resection to clear the margins. If further resection is contraindicated, postoperative radiation therapy is recommended (44).
The role of neoadjuvant therapy in the treatment of T3 tumors is even less clear than the role of adjuvant therapy. There is scarce data to support neoadjuvant chemotherapy or chemoradiotherapy for lung cancer with chest wall invasion. Nonetheless, a recent, multi-institutional, phase II Japanese study of induction chemoradiotherapy followed by surgery in patients with NSCLC involving the chest wall showed that the treatment strategy was safe and effective with a high rate of pathologic response (45).
VATS
Regardless of the surgical approach, either minimally invasive or an open technique, there are 2 goals in the treatment of lung cancer patients with chest wall invasion: to properly stage the mediastinum preoperatively to avoid surgery in N2 disease and to attain negative surgical margins. Complete resection in the context of clear mediastinal lymph nodes is the most important independent predictive factor for survival (2,6,7).
Rationale
In high-volume centers, as surgeons gain experience in VATS lobectomy, they tend to manage more complex cases minimally invasively (46). This reflects the natural instinct of the thoracic surgeon to push the limits without compromising safety or the quality of the oncological resection. In our own series of VATS lobectomies, once the learning curve was achieved, we performed pneumonectomies, bronchoplasties, chest wall resections, and post-chemotherapy resections using a uniportal VATS approach with similar results as thoracotomy (46). Unfortunately, only a few retrospective series address the use of VATS in lung cancer with chest wall invasion (17,21,47-49). The rarity of the clinical presentation, 5% to 8% incidence (5), and the discouraging perception that in this setting a VATS approach cannot be applied or does not offer any benefit in terms of pain control are likely reasons for the limited literature.
Postoperative pain in thoracic surgery is directly related to the number of intercostal nerves injured during surgery and the intensification of central nociception caused by tissue damage and pleural irritation (50-54). In 2016, Bendixen and colleagues demonstrated through a randomized trial that VATS is associated with less postoperative pain and better quality of life than anterolateral thoracotomy for the first year after surgery, suggesting that VATS should be the preferred surgical approach for lobectomy in patients with stage I NSCLC (55). However, there are not any trials comparing VATS versus open approaches in the context of locally invasive lung cancer. Although, it is natural to believe that VATS is less painful, it is essential to validate that basic oncological principles are maintained when a VATS approach is used.
VATS procedures
Early 2000, Widmann and colleagues first reported VATS en bloc lung resection with resection of 2 ribs to treat a lung adenocarcinoma with chest wall infiltration (21). Through a multiple-port VATS approach and with the help of dedicated instruments, the authors described a sublobar procedure followed by chest wall resection. Combining the intrapleural and extrathoracic view, the chest wall resection could be performed in a very precise way (21). Demmy and colleagues described a completely minimally invasive procedure through multiple-port VATS (47). Their procedure starts with the chest wall portion. The intercostal muscles and vessels are dissected and divided with an energy-sealing device; no additional counterincision is performed. Once the compromised chest wall is delimited, the medial aspect of the affected ribs is transected through the 4-cm access incision with a typical rib shear. Subsequently, an endoscopic rib cutter is introduced through the access incision to transect the lateral borders of the ribs. Under direct vision, the macroscopic margins can be accurately defined. The lobectomy is completed last. Over a 7-year period, 15 patients between the ages of 73 and 90 years with NSCLC and chest wall invasion underwent this VATS procedure. There were no conversions to thoracotomy, and median hospital stay was 7 days. However, 90-day mortality was 26.7% for these elderly patients, and median operative time was 500 minutes (48). The benefits of the VATS approach over thoracotomy were highly questioned. Berry and colleagues reported a different minimally invasive strategy, named “hybrid thoracoscopy”, in 12 patients. A standard lobectomy with radical lymphadenectomy is performed first. Then, after adding an intrapleural view to define the compromised chest wall area, a limited counterincision is made above the tumor. In this way, the ribs that need to be transected are precisely identified. The specimen is removed through the counterincision. Complete resection with negative margins was accomplished in all patients, with median hospital stay of 5.5 days, no mortality, and no conversions to thoracotomy. In fact, the postoperative results between this VATS approach and thoracotomy were similar (17).
These VATS techniques have the absence of rib spreading or scapular mobilization in common. Most series of VATS en bloc lobectomy and chest wall resection refer to tumors located posteriorly so the resected chest wall is protected by the scapula and overlying muscles with no need for reconstruction. When indicated, reconstruction can be performed with mesh sutured either thoracoscopically, as described by Abicht and colleagues (20), or directly through the counterincision (17).
Uniportal VATS
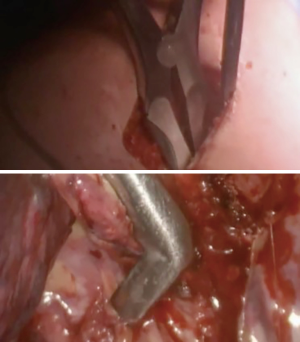
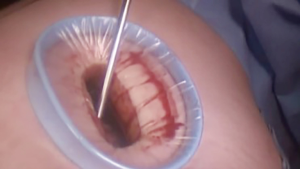
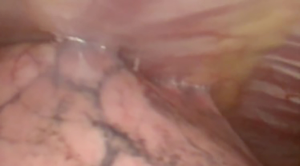
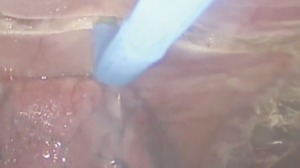
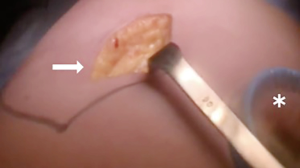
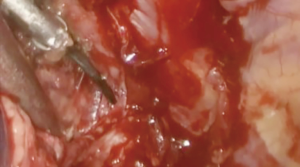
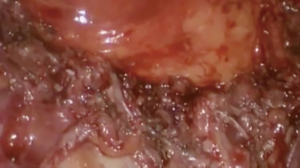
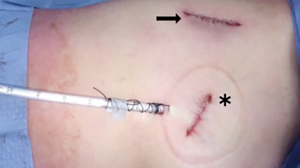
At our institution, we have adopted a uniportal VATS lobectomy with en bloc chest wall resection in patients with T3 tumors that are mediastinal node-negative. We perform the lobectomy with lymphadenectomy first and proceed to the chest wall resection. The utility incision for uniportal VATS is usually placed at the fifth intercostal space between the anterior and mid axillary lines (Figure 1). We find that the adherence to the chest wall keeps counter-traction on the lobar hilum, which facilitates broncho-vascular dissection and completion of the lobectomy (Figure 2). The chest wall margins are delimited with an electrocautery (Figure 3), then the limited counterincision is performed immediately above the chest wall infiltration (Figure 4). Under thoracoscopic guidance and direct vision, the intercostal muscles and neurovascular bundles are divided with an energy-sealing device (Figure 5). The ribs are transected (Figure 6A,B), and the specimen is removed through the counterincision. We believe this combined approach provides a safe and optimal oncological resection. For small defects, with preservation of the external musculature, there is no need for reconstruction (Figure 7). When the incisions are closed, a chest tube for drainage can inserted in the same intercostal space as the utility incision (Figure 8). However, our current preference is to place the chest tube in the posterior corner of the utility incision.
Chest reconstruction
Chest reconstruction restores the rigidity and stability of the chest wall. Reconstruction is important to avoid flail, scapular tip entrapment, and lung herniation and to seal the pleura and prevent infection (56). Anterior chest wall defects are often reconstructed with rigid fixation given their proximity of the heart and vital structures. In general, chest wall defects <5 cm can be managed without reconstruction. Posterior chest wall defects under the scapula do not routinely require reconstruction unless the geometry will cause scapular tip entrapment (24).
There are several controversies regarding the ideal reconstructive material for chest wall defects (41), although the type of reconstruction does not influence postoperative pulmonary morbidity after chest wall resection (41). Two studies showed that a flexible material (such as mesh) can be used to manage most defects (41,56). The use of a rigid prosthesis is not routinely recommended due to the high probability of fracture with the continuous respiratory movements and subsequently the risk of penetration of the surrounding tissues (10).
Conclusions
VATS en bloc lobectomy with chest wall resection for NSCLC is a well-accepted and feasible oncological technique in thoracic surgery. Through a less invasive surgery, patients may benefit from short-term advantages such as less pain, shorter length of hospital stay, and superior preservation of pulmonary mechanics in older and frail patients. Over the last 5 years, several series from high-volume centers have validated VATS approaches as optimal for anatomic lung cancer resection in locally advanced NSCLC, and their acceptance has increased considerably. Surgeons interested in minimally invasive lobectomy with chest wall resection must be perseverant and dedicated to overcome the learning curve and then progress to more complex cases. Additionally, study of long-term outcomes is necessary to better establish the role of VATS in the surgical treatment of locally advanced lung cancer with chest wall involvement.
Acknowledgements
We thank Shannon Wyszomierski for editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Coleman FP. Primary carcinoma of the lung, with invasion of the ribs: pneumonectomy and simultaneous block resection of the chest wall. Ann Surg 1947;126:156-68.12.
- Downey RJ, Martini N, Rusch VW, et al. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg 1999;68:188-93. [Crossref] [PubMed]
- Matsuoka H, Nishio W, Okada M, et al. Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 2004;26:1200-4. [Crossref] [PubMed]
- Detterbeck FC, Boff DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017;151:193-203.
- Grillo HC. Technical consideration in stage III disease: pleural and chest wall involvement. In: Delarue NC, Eschapasse H, editors. International Trends in General Thoracic Surgery. New York, NY: Saunders, 1985;134-8.
- Magdeleinat P, Alifano M, Benbrahem C, et al. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg 2001;71:1094-9. [Crossref] [PubMed]
- Riquet M, Lang-Lazdunski L, Pimperc-Barthes FL, et al. Characteristics and prognosis of resected T3 non-small cell lung cancer. Ann Thorac Surg 2002;73:253-8. [Crossref] [PubMed]
- Albertucci M, DeMeester TR, Rothberg M, et al. Surgery and the management of peripheral lung tumors adherent to the parietal pleura. J Thorac Cardiovasc Surg 1992;103:8-12. [PubMed]
- Burkhart HM, Allen MS, Nichols FC 3rd, et al. Results of en bloc resection for bronchogenic carcinoma with chest wall invasion. J Thorac Cardiovasc Surg 2002;123:670-5. [Crossref] [PubMed]
- Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg 2009;35:450-6. [Crossref] [PubMed]
- Doddoli C, D’Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032-40. [Crossref] [PubMed]
- Elia S, Griffo S, Gentile M, et al. Surgical treatment of lung cancer invading chest wall: a retrospective analysis of 110 patients. Eur J Cardiothorac Surg 2001;20:356-60. [Crossref] [PubMed]
- Facciolo F, Cardillo G, Lopergolo M, et al. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 2001;121:649-56. [Crossref] [PubMed]
- Pitz CC, Brutel de la Riviere A, et al. Surgical treatment of 125 patients with non-small cell lung cancer and chest wall involvement. Thorax 1996;51:846-50. [Crossref] [PubMed]
- Roviaro G, Varoli F, Grignani F, et al. Non-small cell lung cancer with chest wall invasion: evolution of surgical treatment and prognosis in the last 3 decades. Chest 2003;123:1341-7. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Rocco G, Fazioli F, Martucci N, et al. Video-assisted thoracic surgery rib resection and reconstruction with titanium plate. Ann Thorac Surg 2011;92:744-5. [Crossref] [PubMed]
- Nakagiri T, Akashi A, Shigemura N. Thoracoscopic rib resection using a Gigli saw. Ann Thorac Surg 2005;80:755-6. [Crossref] [PubMed]
- Abicht TO, de Hoyos AL. Chest wall resection and reconstruction: a true thoracoscopic approach. Innovations (Phila) 2011;6:399-402. [Crossref] [PubMed]
- Widmann MD, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgery resection of chest wall en bloc for lung carcinoma. Ann Thorac Surg 2000;70:2138-40. [Crossref] [PubMed]
- Lanuti M. Surgical management of lung cancer involving the chest wall. Thorac Surg Clin 2017;27:195-9. [Crossref] [PubMed]
- Hanagiri T, Shinohara S, Takenaka M, et al. Clinical characteristics of resected T3 non-small cell lung cancer characterized by parietal pleural invasion or chest wall invasion. Indian J Surg 2014;76:354-8. [Crossref] [PubMed]
- Lee CY, Byun CS, Lee JG, et al. The prognostic factors of resected non-small cell lung cancer with chest wall invasion. World J Surg Oncol 2012;10:9. [Crossref] [PubMed]
- NCCN. Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 3. 2018.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Tahiri M, Khereba M, Thiffault V, et al. Preoperative assessment of chest wall invasion in non-small cell lung cancer using surgeon-performed ultrasound. Ann Thorac Surg 2014;98:984-9. [Crossref] [PubMed]
- Uhrmeister P, Allmann KH, Wertzel H, et al. Chest wall infiltration by lung cancer: value of thin-sectional CT with different reconstruction algorithms. Eur Radiol 1999;9:1304-9. [Crossref] [PubMed]
- Higashino T, Ohno Y, Takenaka D, et al. Thin-section multiplanar reformats from multidetector-row CT data: utility for assessment of regional tumor extent in non-small cell lung cancer. Eur J Radiol 2005;56:48-55. [Crossref] [PubMed]
- Kawaguchi K, Mori S, Usami N, et al. Preoperative evaluation of the depth of chest wall invasion and the extent of combined resections in lung cancer patients. Lung Cancer 2009;64:41-4. [Crossref] [PubMed]
- Grenier P, Dubary B, Carette MF, et al. Preoperative thoracic staging of lung cancer: CT and MR evaluation. Diagn Interv Radiol 1989;1:23-8.
- Akata S, Kaajiwara N, Park J, et al. Evaluation of chest wall invasion by lung cancer using respiratory dynamic MRI. J Med Imaging Radiat Oncol 2008;52:36-9. [Crossref] [PubMed]
- Vilmann P, Clementse PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2015;48:1-15. [Crossref] [PubMed]
- Hegde PVC, Liberman M. Mediastinal staging endosonographic ultrasound lymph node biopsy or mediastinoscopy. Thorac Surg Clin 2016;26:243-9. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Luzzi L, et al. Lung cancer with chest wall involvement: Predictive factors of long-term survival after surgical resection. Lung Cancer 2006;52:359-64. [Crossref] [PubMed]
- Chapelier A, Fadel E, Macchiarini P, et al. Factors affecting long-term survival after en-bloc resection of lung cancer invading the chest wall. Eur J Cardiothorac Surg 2000;18:513-8. [Crossref] [PubMed]
- Tandberg DJ, Kelsey CR, D’Amico TA, et al. Patterns of failure after surgery for non-small-cell lung cancer invading the chest wall. Clin Lung Cancer 2017;18:e259-65. [Crossref] [PubMed]
- Bayarri CI, Guevara ACL, Martin-Ucar AE. Initial single-port thoracoscopy to reduce surgical trauma during open en bloc chest wall and pulmonary resection for locally invasive cancer. Interact Cardiovasc Thorac Surg 2013;17:32-5. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16. [Crossref] [PubMed]
- Muroako M, Oka T, Tagawa T, et al. Video-assited thoracic surgery lobectomy reduces the morbidity after surgery for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2006;54:49-55.
- Spicer JD, Shewale JB, Antonoff MB, et al. The influence of reconstructive technique on perioperative pulmonary and infectious outcomes following chest wall resection. Ann Thorac Surg 2016;102:1653-9. [Crossref] [PubMed]
- Gao SJ, Corso CD, Blasber JD, et al. Role of adjuvant therapy for node-negative lung cancer invading the chest wall. Clin Lung Cancer 2017;18:169-177.e4. [Crossref] [PubMed]
- Strauss GM, Herndon JE II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group study groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6. 2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Kawaguchi K, Yokoi K, Niwa H, et al. A prospective, multi-institutional phase II study of inductionchemoradiotherapy followed by surgery in patients with non-small cell lung cancer involving the chest wall (CJLSG0801). Lung Cancer 2017;104:79-84. [Crossref] [PubMed]
- Drevet G, Ugalde PA. Uniportal video-assisted thoracoscopic surgery: safety, efficacy and learning curve during the first 250 cases in Quebec, Canada. Ann Cardiothorac Surg 2016;5:100-6. [Crossref] [PubMed]
- Demmy TL, Nwogu CE, Yendamuri S. Thoracoscopic chest wall resection: what is its role? Ann Thorac Surg 2010;89:S2142-5. [Crossref] [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does thoracoscopic surgery decrease the morbidity of combined lung and chest wall resection. Ann Thorac Surg 2015;99:1929-34. [Crossref] [PubMed]
- Gonzalez-Rivas D, Xie B, Yang Y. Uniportal video-assisted thoracoscopic lobectomy with en bloc chest wall resection. J Vis Surg 2015;1:7. [PubMed]
- Alex J, Ansari J, Bahalkar P, et al. Comparison of the immediate postoperative outcome of using the conventional two drains versus a single drain after lobectomy. Ann Thorac Surg 2003;76:1046-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Maniscalco LM. A nondivided intercostal muscle flap further reduces pain of thoracotomy: a prospective randomized trial. Ann Thorac Surg 2008;85:1901-6. [Crossref] [PubMed]
- Ju H, Feng Y, Yang BX, et al. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for postthoracotomy pain control. Eur J Pain 2008;12:378-84. [Crossref] [PubMed]
- Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955-61. [Crossref] [PubMed]
- Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 2007;106:864-7. [Crossref] [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomized controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]