Long-term outcome after resection for recurrent oesophageal cancer
Introduction
Oesophageal cancer is the sixth most common cause of cancer-related mortality worldwide because of its poor prognosis (1). Oesophagectomy with extended lymphadenectomy remains the standard therapy for advanced oesophageal cancer despite the high morbidity rate. However, 30% of patients die after radical oesophagectomy due to recurrence (2). On the other hand, definitive chemoradiotherapy (dCRT) has been one of the treatments for localized oesophageal cancer with curative intent. A phase II clinical trial in Japan demonstrated the efficacy of dCRT as a conservative treatment for clinical stage II–III tumours with a complete response rate of 62% (3). However, 18% of patients after dCRT received salvage oesophagectomy or endoscopic resection for residual disease or locoregional recurrence (3). Whether the therapy used for primary tumour is oesophagectomy or dCRT, tumours recur in many patients, and long-term survival after recurrence cannot be expected.
Generally, chemotherapy or chemoradiotherapy (CRT) has been performed in patients with recurrent oesophageal cancer, but the efficacy of these therapies is limited (4). On the other hand, salvage oesophagectomy for local recurrence after dCRT has been recognized as an established therapy (5,6). Salvage oesophagectomy could offer significant gain in long-term survival compared with second-line CRT (7). However, no reliable studies have demonstrated optimal treatment strategies and long-term outcome for patients with lymph node (LN) or organ recurrence after dCRT. Recently, several investigators have reported comparable benefits of surgical resection for recurrent oesophageal cancer (8,9). However, thus far, the efficacy of surgical resection for LN or organ recurrence of oesophageal cancer has not been sufficiently investigated in studies with large numbers of patients.
The objective of this study was to investigate the long-term outcome of patients who underwent resection for recurrent or residual tumour after surgery or dCRT for primary oesophageal cancer and to reveal appropriate indications for a surgical approach.
Methods
Patients
In the study, resection for recurrent tumour after radical oesophagectomy for primary oesophageal cancer was defined as secondary resection, and resection for recurrent or residual tumour after dCRT was defined as salvage resection. We retrieved patients who underwent secondary or salvage resection at the National Cancer Center Hospital between April 2004 and August 2016. Patients who underwent salvage oesophagectomy were excluded from the outcome analysis. Clinical data were retrospectively collected from our database. Primary tumour was evaluated using the 7th TNM classification published by the Union for International Cancer Control (10).
Oesophagectomy for primary oesophageal cancer
Our standard surgical procedure for advanced primary oesophageal cancer is resection of the oesophagus with three-field LN dissection (11). If the tumour is not suspected to have invaded an adjacent organ, we sometimes select minimally invasive oesophagectomy. A gastric tube is usually used as the reconstruction organ, and the retrosternal route is usually selected.
dCRT for primary oesophageal cancer
The dCRT regimen for primary oesophageal cancer consisted of cisplatin plus 5-fluorouracil combined with radiotherapy. At least two courses of chemotherapy were given, with a dose of 50.4 to 60 Gy radiation. The radiation range was chosen based on the tumour location.
Diagnosis of recurrence
Diagnosis of recurrence was based on physical examinations and computed tomography. Tumour markers and 18F-fluorodeoxy glucose positron emission tomography were used to aid the diagnosis. The final diagnostic criteria for pulmonary metastases were based on histopathological similarity between primary oesophageal cancer and the resected pulmonary specimens.
Surgical inclusion criteria
The indication for second or salvage resection, including such as patient condition and tumour status, was comprehensively decided in our institutional cancer board. The inclusion criteria for LN resection were as follows: tumour involving only one LN or region and no invasion into vital vessels or the tracheobronchial tree. Patients who had one or two pulmonary recurrent tumours were included for surgical resection. The surgical approach chosen for each tumour was decided by the individual surgeons from their experience. Adjuvant chemotherapy and therapy for further recurrence after secondary or salvage resection were not restricted.
Follow up for survival after secondary or salvage resection
Survival after secondary or salvage resection was confirmed by outpatient visit. In addition to physical examinations, computed tomography was periodically performed to detect re-recurrence. For patient who did not visit, we tried to confirm whether the patient was alive or dead by phone.
Statistical analysis
Proportions were compared using the χ2 test, and continuous variables were compared using the Mann-Whitney U-test. Overall survival (OS) was calculated as the period from secondary or salvage resection until death or the date of the last follow-up evaluation. Survival probabilities were calculated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Analyses were performed using SPSS Statistics, version 19. P<0.05 was considered to indicate statistical significance.
Results
Patient characteristics
Between April 2004 and August 2016, our hospital had 882 patients who underwent dCRT for primary oesophageal cancer; 524 achieved complete response and 172 experienced recurrence. Of the 172 recurrent patients, 34 underwent salvage oesophagectomy and 18 underwent salvage endoscopic resection for locoregional recurrence. On the other hand, 1,548 patients underwent oesophagectomy for primary oesophageal cancer, of whom 528 experienced recurrence. During this period, 33 patients underwent salvage resection for recurrent or residual tumour after dCRT (the dCRT group), and 9 patients underwent secondary resection for recurrence after oesophagectomy (the surgery group). The clinicopathological characteristics of the 42 patients who underwent salvage or secondary resection are summarized in Table 1.
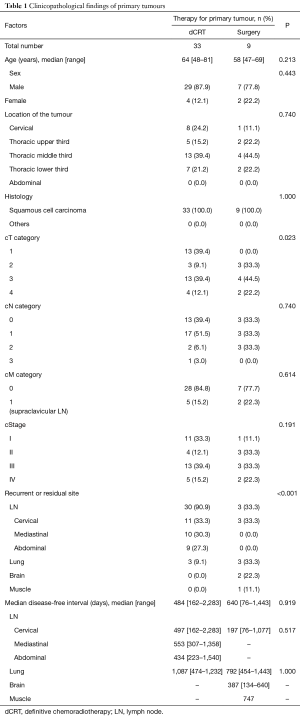
Full table
Mean age was greater in the dCRT group than that in the surgery group; however, this difference was not significant. More cT1 tumours were included in the dCRT group than in the surgery group (39.4% vs. 0.0%, P=0.023). Six patients (66.7%) of the surgery group received neo-adjuvant chemotherapy before surgery for primary tumour. Eight patients (88.9%) of the surgery group underwent complete resection for the primary oesophageal lesion. One patient (11.1%) of the surgery group received adjuvant CRT because a part of the main tumour remained in the left bronchus.
In the dCRT group, 30 patients underwent salvage lymphadenectomy, and 3 patients underwent lung resection. Among the 30 patients who underwent salvage lymphadenectomy, 26 had LN recurrence, and the remaining 4 had residual LNs. Twenty-three (76.7%) patients who underwent salvage lymphadenectomy had recurrent or residual LNs inside the radiation field. In the surgery group, 3 patients underwent secondary lymphadenectomy, 3 underwent lung resection, and 2 underwent brain resection. Among the patients who underwent secondary lymphadenectomy, all recurrences were observed inside the surgical field of the first surgery.
The disease-free interval between the first therapy for the primary tumour and the diagnosis of recurrence ranged from 76 to 2,283 days.
Surgery for recurrent or residual lesions
Cervical LNs were resected via neck incision. Mediastinal LNs were resected via neck incision except in two patients who needed sternum longitudinal incision. Abdominal nodes were resected by laparotomy. Recurrent laryngeal nerves needed to be resected with metastatic LN in eight patients. Complete resection could not be achieved in two patients with mediastinal LN recurrence inside the radiation field because of tumour invasion to the trachea. Postoperative bleeding occurred after left upper lobectomy in one patient, and re-operation was needed. No severe complication was seen in the other 41 cases.
Survival after secondary or salvage resection
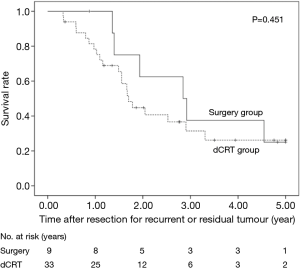
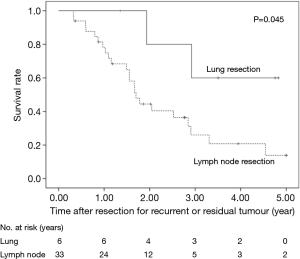
The 5-year OS after secondary or salvage resection was 23.5%. The 5-year OS values of the surgery group and the dCRT group were similar (25.0% vs. 26.2%, P=0.451) (Figure 1). The 5-year OS of patients after lung resection was significantly better than that of patients after LN resection (60% vs. 13.9%, P=0.045) (Figure 2).
Salvage lymphadenectomy
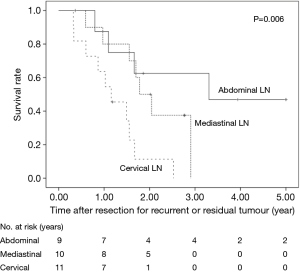
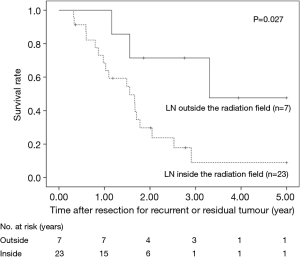
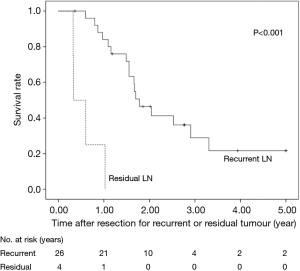
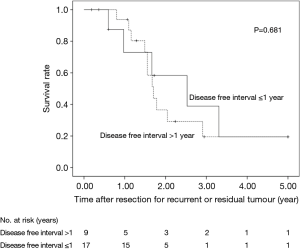
The 5-year OS after salvage lymphadenectomy was 18.7%. The median OS of these cases was 1.7 years. The 5-year OS of patients who underwent salvage abdominal lymphadenectomy was significantly better than that of patients who underwent salvage cervical or mediastinal lymphadenectomy (46.9% vs. 0.0%, P=0.006) (Figure 3). The 5-year OS of patients who underwent salvage resection for LNs outside the radiation field was significantly better than that of patients who underwent salvage resection for LNs inside the radiation field (47.6% vs. 8.9%, P=0.027) (Figure 4). The 5-year OS of patients who underwent salvage resection for recurrent LNs was significantly better than that of patients who underwent salvage resection for residual LNs (21.7% vs. 0.0%, P<0.001) (Figure 5). The 5-year OS of patients who underwent salvage resection for LNs recurred within a year from dCRT had no significant difference from that of patients who underwent salvage resection for LNs recurred after 1 year (19.4% vs. 19.5%, P=0.681) (Figure 6).
Features of long-term survivors
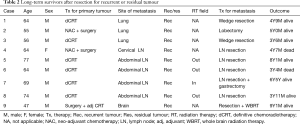
The characteristics of nine patients who survived more than 3 years after secondary or salvage resection are summarized in Table 2. There were 3 survivors of lung resection for solitary recurrence, 1 survivor of secondary cervical lymphadenectomy, 4 survivors of salvage abdominal lymphadenectomy, and 1 survivor of brain resection. Among the 9 long-term survivors, 6 were alive without disease. Three patients achieved 5-year recurrence-free survival, and 1 patient survived more than 5 years with recurrence. Metastatic LNs were detected outside the radiation field in 3 (75%) of the 4 survivors who underwent salvage lymphadenectomy. There was no long-term survivor after salvage resection for residual LN. One patient underwent resection for solitary brain metastasis followed by whole brain radiation therapy (WBRT) and survived more than 5 years without recurrence.
Full table
Discussion
Recently salvage oesophagectomy is recognized as an established therapy for oesophageal cancer, however, salvage lymphadenectomy remains controversial. A few English-language reports have described about safety and short-term survival of lymphadenectomy after dCRT (9,12-14). Doki et al. (12) reported two successful cases who underwent combined removal of the recurrent laryngeal nerve and trachea with LN metastasis after dCRT. Nakajima et al. (13) described four patients who underwent salvage lymphadenectomy and reported that some patients survived longer. Watanabe et al. (9) reported that the median OS of seven patients who underwent salvage lymphadenectomy was 15 months and that no patient survived more than 5 years. However, few previous reports have described long-term survival in studies involving a large number of patients or analysed prognostic factors. This study showed a much better prognosis in 30 patients after salvage lymphadenectomy than that described in previous reports and furthermore described some long-term survivors. Although the number of cases in our study might not be large enough, we consider that this study has important findings from a clinical point of view.
We compared the prognosis according to the site of the metastatic LNs and demonstrated that patients survived significantly longer after salvage abdominal lymphadenectomy than after salvage cervical or mediastinal lymphadenectomy. Previous reports have shown that favourable prognosis was obtained in patients who underwent secondary resection for cervical LN recurrence after oesophagectomy (9,15,16). On the other hand, the efficacy of salvage lymphadenectomy after dCRT seems to be different. Matono et al. (14) reviewed 19 cases who underwent salvage lymphadenectomy after dCRT and showed that the median survival time after salvage abdominal lymphadenectomy was better than that after cervical and/or mediastinal lymphadenectomy (30 vs. 16 months). To our knowledge, our report is the first to describe significant differences in the prognosis of patients after salvage abdominal lymphadenectomy compared with cervical or mediastinal lymphadenectomy. This might be by chance because of low numbers of patients. However, this study suggests an important finding to be proved in the future. Two reasons for favourable prognosis after salvage abdominal lymphadenectomy are indicated. First, en bloc dissection along with left gastric artery is relatively easier than mediastinal LN dissection. Second, abdominal LNs are originally regional LNs of thoracic oesophageal cancer, while cervical LNs have a nature of distant organ.
We demonstrated that there were few long-term survivors after salvage resection for LN that were previously irradiated. On the other hand, long-term survival could be expected in the patients after salvage resection for LN outside the radiation field. The presence of recurrent or residual LNs inside the radiation field indicates that micrometastasis in the field could not be controlled by dCRT. In such cases, complete resection of the extranodal involvement combined with metastatic LNs is quite difficult because of fibrous change of the surround tissue caused by irradiation. Even after achieving the resection of metastatic LN inside the radiation field, the patient might shortly experience further recurrence next to the surgical field. This is partly due to difficulty of en bloc resection with safety margin inside the radiation field. Therefore, salvage resection for LNs inside the radiation field may not provide much benefit in terms of long-term survival.
We furthermore demonstrated more favourable prognosis of patients after salvage lymphadenectomy for recurrent tumour than that of patients after salvage lymphadenectomy for residual tumour. This result is similar to our previous study which showed that tumour recurrence rather than residual tumour was an independent predictive factor for increased survival after salvage oesophagectomy (17). A complete response to dCRT means not only complete remission of the primary lesion and the metastatic regional LNs but also success in treating potential intramural and regional metastases. Therefore, long-term survival could be expected after salvage resection for recurrent LN.
In addition, this study revealed favourable prognosis after secondary resection for solitary distant recurrence. Four reports have studied the long-term prognosis after resection for the pulmonary recurrence of oesophageal cancer and reported 5-year survival rates of 29.6–43.3% (8,18-20). Our results showed better prognoses than previous reports. This might be partly because of the relatively small pulmonary lesions or the low number of patients in our study. Although it is difficult to distinguish metastatic oesophageal cancer from primary squamous cell lung cancer even based on a pathological diagnosis, our results suggest the efficacy of resection for solitary lung recurrence. In the meantime, brain metastasis from oesophageal cancer are rare, therefore efficacy of surgical resection remains unclear. Weinberg et al. (21) reported relatively favourable survival in patients with a single brain lesion who underwent resection followed by WBRT. We encountered a long-term survivor with more than 5-year survival without recurrence after similar therapy. This combined strategy might improve the prognosis of patients with brain recurrence.
There are several limitations to our study. First, this is a retrospective study conducted at a single institution. Second, selection bias for the surgical treatment existed. Third, the relatively favourable prognosis after salvage lymphadenectomy might have occurred because of the patient characteristics of the dCRT group, which was comprised of many stage I patients. Therefore, a large-scale prospective study involving many institutions is needed.
Conclusions
In conclusion, the use of the appropriate surgical approach might improve the prognosis of patients with abdominal LN recurrence, LN recurrence outside the radiation field, or solitary lung recurrence of oesophageal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional ethics board of National Cancer Center Hospital (No. 2017-061), and the requirement for informed patient consent was waived due to the retrospective nature of this study.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2009. Esophagus 2016;13:110-37. [Crossref] [PubMed]
- Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684-90. [Crossref] [PubMed]
- Ma X, Zhao K, Guo W, et al. Salvage lymphadenectomy versus salvage radiotherapy/chemoradiotherapy for recurrence in cervical lymph node after curative resection of esophageal squamous cell carcinoma. Ann Surg Oncol 2015;22:624-9. [Crossref] [PubMed]
- Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009;137:49-54. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Salvage Surgery After Chemoradiotherapy in the Management of Esophageal Cancer: Is It a Viable Therapeutic Option? J Clin Oncol 2015;33:3866-73. [Crossref] [PubMed]
- Kumagai K, Mariosa D, Tsai JA, et al. Systematic review and meta-analysis on the significance of salvage esophagectomy for persistent or recurrent esophageal squamous cell carcinoma after definitive chemoradiotherapy. Dis Esophagus 2016;29:734-9. [Crossref] [PubMed]
- Shiono S, Kawamura M, Sato T, et al. Disease-free interval length correlates to prognosis of patients who underwent metastasectomy for esophageal lung metastases. J Thorac Oncol 2008;3:1046-49. [Crossref] [PubMed]
- Watanabe M, Mine S, Yamada K, et al. Outcomes of lymphadenectomy for lymph node recurrence after esophagectomy or definitive chemoradiotherapy for squamous cell carcinoma of the esophagus. Gen Thorac Cardiovasc Surg 2014;62:685-92. [Crossref] [PubMed]
- International Union Against Cancer. Oesophagus including oesophagogastric junction. In: Sobin LH, Gospodarowicz MK, Wittekind C (ed.) TNM Classification of Malignant Tumours. West Sussex, UK: WileyBlackwell, 2009;66-72.
- Igaki H, Kato H, Tachimori Y, et al. Prognostic evaluation of patients with clinical T1 and T2 squamous cell carcinomas of the thoracic esophagus after 3-field lymph node dissection. Surgery 2003;133:368-74. [Crossref] [PubMed]
- Doki Y, Yasuda T, Miyata H, et al. Salvage lymphadenectomy of the right recurrent nerve node with tracheal involvement after definitive chemoradiation therapy for esophageal squamous cell carcinoma: report of two cases. Surg Today 2007;37:590-5. [Crossref] [PubMed]
- Nakajima M, Domeki Y, Satomura H, et al. Salvage lymphadenectomy for recurrent esophageal cancer after chemoradiotherapy. Int Surg 2014;99:452-7. [Crossref] [PubMed]
- Matono S, Fujita H, Tanaka T, et al. Salvage lymphadenectomy without esophagectomy is an option for recurrent or residual lymph nodes after definitive chemoradiotherapy for esophageal cancer. Esophagus 2014;11:197-203. [Crossref]
- Motoyama S, Kitamura M, Saito R, et al. Outcome and treatment strategy for mid- and lower-thoracic esophageal cancer recurring locally in the lymph nodes of the neck. World J Surg 2006;30:191-8. [Crossref] [PubMed]
- Yano M, Takachi K, Doki Y, et al. Prognosis of patients who develop cervical lymph node recurrence following curative resection for thoracic esophageal cancer. Dis Esophagus 2006;19:73-7. [Crossref] [PubMed]
- Wang S, Tachimori Y, Hokamura N, et al. Prognostic analysis of salvage esophagectomy after definitive chemoradiotherapy for esophageal squamous cell carcinoma: The importance of lymphadenectomy. J Thorac Cardiovasc Surg 2014;147:1805-11. [Crossref] [PubMed]
- Ichikawa H, Kosugi S, Nakagawa S, et al. Operative treatment for metachronous pulmonary metastasis from esophageal carcinoma. Surgery 2011;149:164-70. [Crossref] [PubMed]
- Kobayashi N, Kohno T, Haruta S, et al. Pulmonary metastasectomy secondary to esophageal carcinoma: long-term survival and prognostic factors. Ann Surg Oncol 2014;21:S365-9. [Crossref] [PubMed]
- Kanamori J, Aokage K, Hishida T, et al. The role of pulmonary resection in tumors metastatic from esophageal carcinoma. Jpn J Clin Oncol 2017;47:25-31. [Crossref] [PubMed]
- Weinberg JS, Suki D, Hanbali F, et al. Metastasis of esophageal carcinoma to the brain. Cancer 2003;98:1925-33. [Crossref] [PubMed]


