Multi-level analysis of bacteria isolated from inpatients in respiratory departments in China
Introduction
Respiratory tract infections are the most common infectious disease of respiratory tract, and irrational use of antimicrobial agents has introduced serious antibiotic resistance (1-4). In United State, although there were strict regulations for the use of antimicrobial agents for respiratory infections, such as bronchitis, pharyngitis, sinusitis and common cold, 41% of antimicrobial agents were prescribed for respiratory diseases (5,6). In China, the irrational use of antibiotics was still severe. Researches revealed that over 50% outpatients, including 80% outpatients with upper respiratory tract infections were prescribed antibiotics (7,8). This problem was more significant in the primary hospitals, as less than 40% outpatients and less than 25% inpatients conformed to the principle of antibiotics; more than 93% patients with upper respiratory tract infections, most of which were supposed to be viral infections, were prescribed antibiotics (9). To date, China has become one of largest consumer of antibiotics in the world (10). A multicenter epidemiology survey on hospital acquired pneumonia revealed that the detection rates of carbapenem-resistant Acinetobacter baumannii (CRAB) and Pseudomonas aeruginosa (CRPA) were 78.9% and 70.7%, respectively, while the rate of methicillin-resistant Staphylococcus aureus is 87.8% (MRSA) (11). China is a vast country with a huge number of medical institutions. Mainly due to the disparities in socioeconomic development, the situation for clinical antibiotic usage and its management varied in different regions and amongst medical institutions of different level (12). However, the epidemiological data of multi-drug resistant (MDR) strains from the department of respiration is limited. Therefore, this study aims to analyze the distribution and epidemic characteristics of the target MDR isolates from department of respiration in China via different levels and to provide the basis for the antibiotic regulation.
Methods
Bacteria source
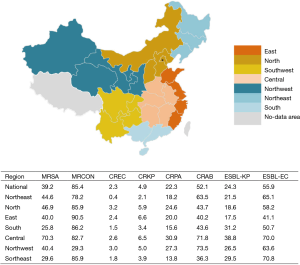
Based on the data of China Antimicrobial Resistance Surveillance System (CARSS) in 2015, 50,417 non-duplicated bacterial strains from inpatient in respiratory departments from 91 general hospitals (9 in Northeast China, 16 in the north region of China, 14 in East China, 9 in southern China, 15 in Central China, 14 in Northwest China, 14 in Southwest China) in seven regions of China were enrolled in the study (Figure 1). Briefly, 90.2% (45,491/50,417) strains were obtained from 63 tertiary hospitals, and 9.8% (4,926/50,417) strains from 28 secondary hospitals. Meanwhile, 6.2% (3,129/50,417) strains were isolated from respiratory intensive care units (RICUs) and 93.8% (47,288/50,417) were isolated from non-ICU respiratory department. Furthermore, age groups were classified as: neonatal (age ≤28 days), pediatric (age from 29 days to 14 years old), adult (age from 15 to 65 years old) and geriatric groups (age >65 years old). The number of isolates from geriatric group accounted for 46.0% (23,177/50,417), followed by adult group (29.9%, 15,092/50,417) and pediatric group (24.0%, 12,112/50,417).
Species identification and quality control
Species identification and antimicrobial susceptibility testing were performed in locally. The species identification was performed by standard biochemical methods including API 20E system, Vitek 2 compact (bioMérieux, Marcy l’Etoile, France) and Matrix-assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS, bioMérieux, Marcy l’Etoile, France), etc. The antimicrobial susceptibility test was interpreted according to clinical and laboratory standards institute (CLSI) 2015 guidelines (13). Quality control of the dataset was conducted by Peking Union Medical College Hospital, the national quality control center of antimicrobial resistance.
Statistically analysis
Distribution of methicillin-resistant S. aureus (MRSA), carbapenem-resistant Escherichia coli (CREC), carbapenem-resistant Klebsiella pneumoniae (CRKP), CRPA, CRAB and extended-spectrum β-lactamases-producing E. coli (ESBL-EC) and K. pneumoniae (ESBL-KP) were analyzed by the WHONET 5.6 software. Statistical analysis was performed by IBM SPSS software (version 16.0; IBM SPSS Inc., New York, USA). Categorical variables were compared using the Chi-square test. P values of 0.05 were considered significant.
Results
Prevalence of different bacterial species in respiratory departments in China
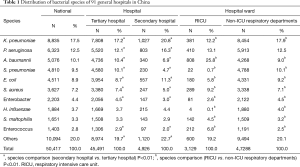
Overall, a total of 50,417 non-duplicated isolates were collected from the respiratory department of 91 general hospitals, with the top four species being K. pneumoniae (17.5%, 8,835/50,417), P. aeruginosa (12.5%, 6,323/50,417), A. baumannii (10.1%, 5,076/50,417) and S. pneumoniae (9.5%, 4,810/50,417). Meanwhile, the top four species in secondary hospitals were K. pneumoniae (20.8%, 1,027/4,926), P. aeruginosa (16.3%, 803/4,926), E. coli (11.3%, 557/4,926) and A. baumannii (6.9%, 340/4,926). Additionally, the leading top four species in RICU were A. baumannii (25.8%, 808/3,129), P. aeruginosa (13.1%, 410/3,129), K. pneumoniae (12.2%, 381/3,129) and S. aureus (9.2%, 289/3,129) (Table 1).
Full table
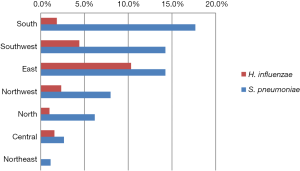
For the detection rate of fastidious bacteria in various regions, the highest isolation rate of Streptococcus pneumoniae was in the South region (17.7%, 837/4,736), while the lowest was in the Northeast (1.1%, 55/4,853). The highest detection rate of Haemophilus influenzae was found in East China (10.3%, 1,081/10,463) (Figure S1).
Specimen types
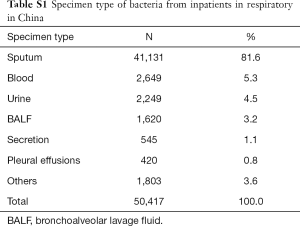
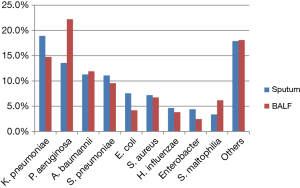
The main specimen types of the collection were sputum (81.6% 41,131/50,417), followed by blood sample (5.3%, 2,649/50,417), urine (4.5%, 2,249/50,417) and bronchoalveolar lavage fluid (BALF) (3.2%, 1,620/50,417) (For detail information of specimen type and the distribution of MDR bacteria by specimens, please refer to Tablea S1,S2). In BALF and sputum specimens, the top four species were similar: K. pneumonia (18.9% and 14.8%, respectively), P. aeruginosa (13.6% and 22.2%, respectively), A. baumannii (11.3% and 11.9%, respectively) and S. pneumonia (11.1% and 9.6%, respectively). The proportion of non-fermentative bacteria, i.e., P. aeruginosa and S. maltophilia in BALF was higher than that in sputum 22.2% vs. 13.6% and 6.2% vs. 3.4%, respectively, while the rate of K. pneumoniae and E. coli in sputum was higher than that in BALF (18.9% vs. 14.8% and 7.6% vs. 4.2%, respectively) (all P<0.05) (Figure 2).
Full table
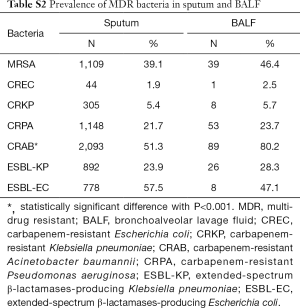
Full table
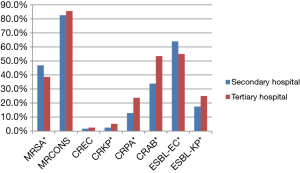
Distribution of MDR bacteria by hospital ranks
The overall detection rate of MRSA in the secondary hospitals was higher than that in the tertiary hospitals (46.8% vs. 38.6%) (P=0.013). Additionally, the rates of CRKP, CRPA, CRAB and ESBL-KP in the tertiary hospitals were higher than that in secondary hospitals (5.2% vs. 2.5%, 23.8% vs. 12.8%, 53.5% vs. 33.9% and 25% vs. 17.5%), respectively (all P<0.01). The detection rate of ESBL-KP in tertiary hospitals was lower than that in secondary hospital (55.0% vs. 63.9%) (P=0.01) (Figure 3).
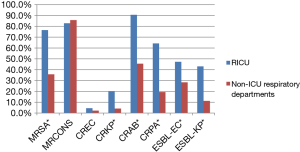
Distribution of MDR bacteria in RICUs and non-ICU respiratory departments
The detection rates of MRSA, CRKP, CRAB, CRPA, ESBL-EC and ESBL-KP in RICUs were significantly higher than that in non-ICU respiratory departments (76.5% vs. 35.7%, 20.1% vs. 4.1%, 90.6% vs. 45.5%, 64.2% vs. 19.3%, 47.2% vs. 28.3% and 43.0% vs. 11.2%, respectively) (all P<0.01) (Figure 4).
Regional distribution of MDR bacteria in respiratory departments
The national average detection rate of MRSA was 39.2% (1,316/3,356), of which the highest rate was detected in Central region (70.3%, 237/337), and lowest in Southern region (25.8%, 163/632). The national average detection rate of CREC was 2.3% (99/4,215), with rates fluctuating at a low level (0.4–3.0%) in varied regions. The national average detection rate of CRKP was 4.9% (408/8,412), with the highest rate in East (6.6%, 75/1,144) and the lowest in Northeast region (2.1%, 28/1,327). The national average detection rate of CRPA was 22.3% (1,361/6,091), with highest rate in Center China (30.9%, 376/1,218) and the lowest in Southwest region (13.8%, 102/737). The national average detection rate of CRAB was 52.1% (2,368/4,546), with high rates in Northwest (73.5%, 391/532) and Central region (71.8%, 487/678). The national average detection rate of ESBL-KP was 24.3% (1,080/4,443), with most prevalence (38.8%, 241/621) in Central and lowest in East region (17.5%, 172/984). The national average detection rate of ESBL-EC was 55.9% (1,286/2,301), with high rate in Southwest (70.8%, 243/343) and Central China (70.0%, 217/310) (Figure 1).
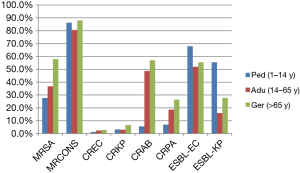
Distribution of MDR bacteria in respiratory departments by age groups
The detection rate of MRSA in geriatric group (57.9%, 608/1,050) was significant higher than that in adult (36.8%, 285/775) and pediatric group (27.7%, 423/1,529) (all P<0.01), whilst no MRSA strains were found in neonatal group. Additionally, the rates of CREC in geriatric group (2.8%, 60/2,119) were higher than that in pediatric group (1.3%, 12/951) (P=0.008). Similarly, the rates of CRKP in geriatric group (6.6%, 284/ 4,301) were higher than that in adult (3.0%, 97/3,225) and pediatric group (3.2%, 27/849) (all P<0.001), whilst no CRKP strain were found in neonatal group. The detection rates of ESBL-EC and ESBL-KP in pediatric group (68.2% and 55.3%, respectively) were higher than that in geriatric group (54.2% and 27.1%, respectively) and adult group (51.1% and 15.1%, respectively) (all P<0.001). For non-fermentative bacteria, the rates of CRAB and CRPA in geriatric group (57.0% and 26.3%, respectively) were higher than that in adult (48.7% and 18.6%, respectively) and pediatric group (5.5% and 6.9%, respectively) (all P<0.001) (Figure 5).
In vitro susceptibility of specific agents
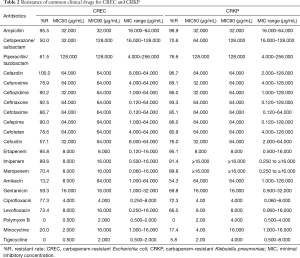
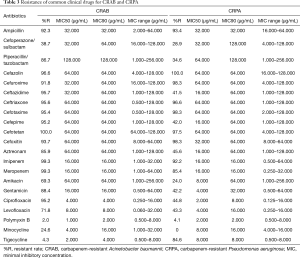
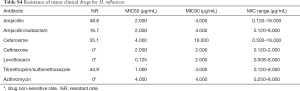
For the major clinical antimicrobial agents against CRKP, all of strains (30/30) were susceptible to polymyxin B, with minimal inhibitory concentration (MIC)90 4.000 µg/mL, whilst 5.8% (3/52) of the strains were resistant to tigecycline, with MIC90 4.000 µg/mL (Table 2). For susceptibility of CRAB, the resistance rate of polymyxin B and tigecycline were 2.0% (4/205; MIC90 2.000 µg/mL) and 4.3% (22/515; MIC90 4.000 µg/mL), respectively. As for CRPA, the resistance rate of Polymyxin B was 4.1% (10/243), with MIC90 2.000 µg/mL (Table 3). For the major clinical antimicrobial agents against fastidious bacteria (Streptococcus Haemophilus influenzae), ESBL-EC and ESBL-KP, please refers to supplementary material Tables S3-S5).
Full table
Full table

Full table
Full table
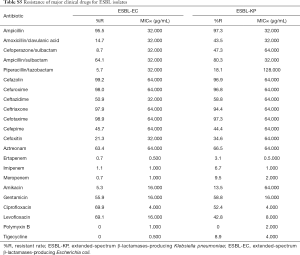
Full table
Discussion
MDR has been a public health problem. In order to cope with the threat posed by MDR bacteria with high resistance against major clinical antibiotics, WHO first enumerated twelve MDR bacteria in 2017 (14), including the ones associated with the common community-acquired pneumonia and Hospital acquired pneumonia in the respiratory departments in China. Therefore, multi-level epidemiological analysis of isolates from respiratory department in China is significant and instructive, not only for a better understanding of antimicrobial resistance in China, but also for the improvement of the rational use of antimicrobial agents.
In our study, the distribution of species from qualified sputum specimens were basically consistent with BALF, with consistency for top four species, i.e., K. pneumonia, P. aeruginosa, A. baumannii and S. pneumonia. Interestingly, the proportion of P. aeruginosa and S. maltophilia in BALF was significantly higher than that in sputum. The reasons for this phenomenon may be as follow: (I) due to the differences sensitivity rate of sputum culture of varied bacteria (15), moderate consistency was found between sputum and BALF (16); (II) meanwhile, invasive procedure, including BALF, was commonly conducted in qualified hospitals and patients from these hospitals had received antibiotic treatment before taking sample, which resulted in the increase of MDR strains including P. aeruginosa and S. maltophilia (15). Furthermore, the high inspection rate of sputum culture from numerous local primary hospitals before prescribing antimicrobial agents may result in the increase of specie types and the relative decrease of MDR bacteria in sputum, i.e., P. aeruginosa and S. maltophilia. Therefore, in view of the recognition of qualified semi quantitative sputum culture by Infectious Diseases Society of America (IDSA) and the advance of microbial laboratory in China, high quality sputum specimens and sterile specimens obtained by bronchoscopy are essential for the etiology diagnosis.
For hospital distribution of varied MDR strains, the detection rates of CRKP, CRAB, and CRPA in tertiary hospitals were significant higher than the secondary hospitals. There were two reasons for this phenomenon. On one hand, due to the irrational use of antibiotics and the selective pressure of antibiotics in primary hospitals or secondary hospitals, the multidrug-resistant bacteria might be likely emerged before the submission into tertiary hospitals and prescribed a more broad-spectrum antibiotic in tertiary hospital. On the other hand, the irrational use and overuse of antibiotics still existed in some tertiary hospitals in the well-developed cities, which was one of the important factors for the emergence and prevalence of MDR bacteria (8,17). Notably, in this study, the detection rates of ESBL-EC and ESBL-KP were high in the secondary hospitals and the rate of ESBL-EC in secondary hospitals was significant higher than that in the tertiary hospitals. This phenomenon is consistent with another epidemiological study on community-acquired bloodstream infections in China, indicating a high prevalence of ESBL-EC in Chinese community, which was up to 51.0% (18). The prevalence of ESBL-EC and ESBL-KP in secondary hospitals might be associated with the high utilization of cephalosporins. Compared to the tertiary hospitals in well-developed cities, the use and misuse of antibiotics were more serious in primary hospitals and community hospitals in less-developed areas. Some studies showed that the utilization rate of antibiotics for outpatient in primary hospitals and secondary hospitals were 53.4% and 49.2% respectively, higher than the tertiary hospital (47.1%) (8,9,19). As for the outpatients with upper respiratory tract infections, the antibiotic usage rate in secondary hospitals was 84.8% and over 90% of the patients with acute upper respiratory infections were treated with antibiotics, among which 28% of the patients were treated with cephalosporins (7,9). In addition, in the livestock area with high ESBL detection rate, including Northeast and West regions, animal-mediated ESBL infections were associated with the high prevalence of ESBL in local primary hospitals. Our study also revealed that a high detection rate of ESBL-EC and ESBL-KP in pediatric population. This phenomenon might be associated with the high utilization rate of cephalosporin antibiotics in pediatric outpatients, as the usage of cephalosporins, especially the third generation cephalosporin, was one of the risk factors for children infected with ESBL-KP and ESBL-EC (20). Therefore, it is particularly important for primary hospitals to strengthen the standardized and rational use of antibiotics in multiple links.
In this study, the distribution of multi-drug resistant bacteria in RICU was significantly higher than that in the non-ICU respiratory departments. On one hand, the patients in RICU with severe disease, including cancer, kidney failure, heart disease, chronic obstructive pulmonary disease, etc., were commonly prescribed broad-spectrum antibiotics for empirical therapy, posing the risk of MDR bacterial infections to patients (21-23). On the other hand, the risk of ventilator-associated pneumonia (VAP) increased due to the prolonged hospitalization in ICU and routine invasive procedures such as intubation and respiratory support. The IDSA 2016 guidelines also point out that, the usage of intravenous broad-spectrum antibiotics is one of the risk factors for MDR infections in VAP patients, making VAP more difficult to treat (23,24). Meanwhile, the special environment of the ICU departments provided convenient conditions for the transmission of MDR clones, including CC22 of CRAB, ST11 of CRKP, ST239 of MRSA, etc., which poses a great risk for ICU patients (25-28). Therefore, in view of the high prevalence of MDR bacterial in RICU, the establishment of RICU monitoring system, including the monitoring of MDR clones with high pathogenicity, was an urgent need to provide relevant basis for clinical treatment and for related control measures making.
Compared with the national data in 2014, the detection rate of MDR strains in 2015, including MRSA (39.2% vs. 44.6%), CRKP (4.9% vs. 10.5%), CRPA (22.3% vs. 26.6%), CRAB (52.0% vs. 62.4%) and ESBL-KP (24.3% vs. 29.9%) were lower, while the rate of CREC and ESBL-EC were similar (29). Notably, the AMR in Central region was serious, with the highest rates of MRSA, CRPA, ESBL-KP and second highest rates of CRKP, CRAB and ESBL-EC. Studies on the usage of antibiotics in China revealed that 87.3% outpatients in respiratory departments were prescribed one antibiotics, which is significantly higher than the west China and the east China (7). Meanwhile, it is worth noticing that the high detection rate of ESBL-EC was found not only in the Central region, but also in Southwest, Northwest and Northeast region of China whereas possesses prosperous livestock industry. And this finding basically consistent with the results of another ESBL bloodstream infection research (18). The irrational use of antibiotics in these areas promotes the transmission of ESBL-producing strains between humans and animals (18,19,30). Therefore, strengthening the management of antibiotic use and continuous monitoring of MDR bacteria in these drug-resistant epidemic areas were highly needed
Our study also has some limitations. On one hand, since not all the respiratory departments of general hospitals were enrolled in this study, the detection rates of resistant bacteria can not accurately reflect the resistance in various regions. However, the overall detection rates of MDR bacteria from the respiratory departments in this study remain a high degree of consistency with the available national data. On the other hand, contamination of sputum was unavoidable completely, which would slightly affect the regional distribution of MDR bacteria.
Conclusions
In China, the predominant bacterial pathogens in the respiratory ward were Enterobacteriaceae and non-fermentative bacteria. High prevalence of ESBL-EC and ESBL-KP isolated from lower respiratory tract (LRT) was revealed in primary hospitals and pediatric patients.
Acknowledgements
We grateful thank members of China Antimicrobial Resistance Surveillance System for data collection, and stuffs of national quality control center in Peking Union Medical College Hospital for the quality control.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81471989).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sharifi-Rad J, Belkum AV, Fallah F, et al. Rising Antimicrobial Resistance in Iran. Der Pharmacia Lettre 2016;8:31-3.
- Centers for Disease Control and Prevention (CDC). Antibiotic resistance threat in United State, 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/
- Sharifi-Rad M, Iriti M, Sharifi-Rad M, et al. Anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of Rubiaceae, Fabaceae and Poaceae plants: A search for new sources of useful alternative antibacterials against MRSA infections. Cell Mol Biol (Noisy-le-grand) 2016;62:39-45. [PubMed]
- Salehi B, Mehrabian S, Ahmadi M. Investigation of antibacterial effect of Cadmium Oxide nanoparticles on Staphylococcus Aureus bacteria. J Nanobiotechnology 2014;12:26. [Crossref] [PubMed]
- Harris AM, Hicks LA, Qaseem A, et al. Appropriate Antibiotic Use for Acute Respiratory Tract Infection in Adults: Advice for High-Value Care From the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2016;164:425-34. [Crossref] [PubMed]
- Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother 2014;69:234-40. [Crossref] [PubMed]
- Li J, Song X, Yang T, et al. A Systematic Review of Antibiotic Prescription Associated With Upper Respiratory Tract Infections in China. Medicine (Baltimore) 2016;95:e3587. [Crossref] [PubMed]
- Yin X, Song F, Gong Y, et al. A systematic review of antibiotic utilization in China. J Antimicrob Chemother 2013;68:2445-52. [Crossref] [PubMed]
- Wang J, Wang P, Wang X, et al. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med 2014;174:1914-20. [Crossref] [PubMed]
- Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742-50. [Crossref] [PubMed]
- Zhao T, Liu Y, Cao B, et al. Prospective multicenter study of pathogen distributions in early-onset and late-onset hospital-acquired pneumonia in china. Antimicrob Agents Chemother 2013;57:6404-5. [Crossref] [PubMed]
- Xu A, Zheng B, Xu YC, et al. National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infect 2016;22 Suppl 1:S1-8. [Crossref] [PubMed]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute 2015; CLSI document M100-S25.
- Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018;18:318-27. [Crossref] [PubMed]
- Fukuyama H, Yamashiro S, Kinjo K, et al. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective observational study. BMC Infect Dis 2014;14:534. [Crossref] [PubMed]
- Wood AY, Davit AJ 2nd, Ciraulo DL, et al. A prospective assessment of diagnostic efficacy of blind protective bronchial brushings compared to bronchoscope-assisted lavage, bronchoscope-directed brushings, and blind endotracheal aspirates in ventilator-associated pneumonia. J Trauma 2003;55:825-34. [Crossref] [PubMed]
- Wang YY, Du P, Huang F, et al. Antimicrobial prescribing patterns in a large tertiary hospital in Shanghai, China. Int J Antimicrob Agents 2016;48:666-73. [Crossref] [PubMed]
- Quan J, Zhao D, Liu L, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother 2017;72:273-80. [Crossref] [PubMed]
- Wang H, Li N, Zhu H, et al. Prescription pattern and its influencing factors in Chinese county hospitals: a retrospective cross-sectional study. PLoS One 2013;8:e63225. [Crossref] [PubMed]
- Du B, Long Y, Liu H, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med 2002;28:1718-23. [Crossref] [PubMed]
- Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005;128:3854-62. [Crossref] [PubMed]
- Lee H, Park JY, Lee T, et al. Intermediate risk of multidrug-resistant organisms in patients who admitted intensive care unit with healthcare-associated pneumonia. Korean J Intern Med 2016;31:525-34. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-111. [Crossref] [PubMed]
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903. [Crossref] [PubMed]
- Li H, Zhang J, Liu Y, et al. Molecular characteristics of carbapenemase-producing Enterobacteriaceae in China from 2008 to 2011: predominance of KPC-2 enzyme. Diagn Microbiol Infect Dis 2014;78:63-5. [Crossref] [PubMed]
- Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiology Reviews 2011;35:736-55. [Crossref] [PubMed]
- Xiao M, Wang H, Zhao Y, et al. National surveillance of methicillin-resistant Staphylococcus aureus in China highlights a still-evolving epidemiology with 15 novel emerging multilocus sequence types. J Clin Microbiol 2013;51:3638-44. [Crossref] [PubMed]
- Fu Y, Zhou J, Zhou H, et al. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother 2010;65:644-50. [Crossref] [PubMed]
- Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect 2016;22 Suppl 1:S9-14. [Crossref] [PubMed]
- Gibson MK, Wang B, Ahmadi S, et al. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat Microbiol 2016;1:16024. [Crossref] [PubMed]


