The Society for Translational Medicine: the assessment and prevention of venous thromboembolism after lung cancer surgery
Introduction
Cancer is an independent major risk factor for venous thromboembolism (VTE), which is the second leading cause of death in medically and surgically treated patients with cancer (1-5). The association between VTE and lung cancer has been reported more than 20 years ago (6,7).
Lung cancer is the leading cause of death in China for both men and women, with over 626,000 deaths in 2014 in China (8). VTE after lung cancer surgery occurs at a high incidence (9,10).
Featured by insidious onset and high case-fatality rate, VTE is one of the main causes of perioperative deaths and is also a key contributor to unexpected deaths in hospitals (1,11,12). In lung cancer patients, effective prevention measures not only decrease the risk of VTE and alleviate the patients’ pain but also improve the long term survival and lower the medical costs (2,13).
Therefore, the prevention VTE in lung cancer surgery patients represents major challenges for thoracic surgeons in daily practice. In recent years, there has been increasing attention to the phenomenon of VTE in lung cancer surgery (14). Although several national and international guidelines for the prevention and treatment of VTE in cancer patients have been published in the past, however none was specific for lung cancer (4,5,15-25). Accordingly and in view of the importance of this issues, The Society for Translational Medicine with the collaboration of China National Research Collaborative Group on Venous Thromboembolism in Thoracic Surgery, set up a working group to develop the Assessment and Prevention of Venous Thromboembolism after Lung Cancer Surgery. The working group comprised 38 experts from various specialties and sub-specialties (thoracic surgery, respiratory medicine, vascular surgery, pharmacology), including eight international panelists, as well as one nurse. The aim of this article is to provide more accurate knowledge and to improve and standardize the prevention of VTE after lung cancer surgery.
Basic concepts
VTE: abnormal clotting of blood in veins leads to complete or incomplete obstruction of blood vessels. VTEs are venous blood return-related disorders. VTE is manifested as deep vein thrombosis (DVT) and/or pulmonary embolism (PE), which represent two clinical manifestations of VTE at different sites in the body at different stages (5,10,26,27). DVT, when blood clots occur within the deep veins, is especially prevalent in the deep veins of the lower limbs, but can occur all over the body. It often occurs after major orthopedic surgery, but can also occur following lung cancer surgery. DVT in lower limbs (popliteal vein or its proximal parts) is considered a main source of pulmonary thromboembolism. Prevention of DVT can reduce the risk of PE (5). PE, the occlusion of the pulmonary artery or its branches by thrombus from the venous system or the right heart, is a pulmonary circulation and respiratory function disorder that can be fatal. PE is one of the major causes of perioperative death among lung cancer patients (5).
Epidemiology of VTE
VTE is a common disease that poses serious threats to cancer patients, especially in patients who have received chemotherapy and/or surgery (26,28). The incidence of VTE in cancer patients who have been hospitalized for the first time is about 3–12%, depending on the site of the malignancies (28,29). Approximately 20% of all VTE events occur in patients with cancer (12) and more than 20% of cancer patients will be affected by VTE before death (30). In a survey of clinical trials of thromboprophylaxis in surgical patients with cancer, the average incidence of DVT in untreated patients was 29% (28).
In another study that had enrolled 17,284 patients with solid tumors, the incidence of VTE was 12.6% within 12 months after outpatient chemotherapy (31). Both DVT and PE have high case-fatality rates in their early stages (3.8% and 38.9%, respectively), thus being the second leading cause of deaths among patients with malignant tumors (32,33).
It has been reported both in China and abroad that the incidence of VTE after lung cancer surgery is 7.3–13.9% (1,11,12,34-36). Dentali et al. reported that the incidence of VTE after surgery for lung cancer was 7.4%, with the peak occurring within 7 days after surgery (1). In a prospective cohort using computerized tomography pulmonary angiogram (CTPA) and lower limb venous compression ultrasound one month after surgery to measure the primary outcome, the incidence of post-operative VTE was 12.1%, of which 80% of the VTE events were PE, and only 21% were symptomatic (37). Zhang et al. described the high prevalence of VTE in Chinese patients with newly diagnosed lung cancer. VTE events occurred in 89 (13.2%) of the 673 patients enrolled in the study (35). A retrospective study on resected specimens obtained from lung cancer patients found that 59.6% of lung cancer patients experienced thromboembolic events, among which 42.9% were pulmonary arterial embolism and 57.1% pulmonary venous embolism (38). Ziomek et al. analyzed 77 patients who underwent lung resection surgery for lung cancer. They found that 20 patients (26%) had a thromboembolic episode in the postoperative period (15 cases of DVT and 5 cases of PE) (39).
In a most recent single-center, prospective cohort study, Li et al. found that the overall incidence of VTE was 13.9% (48 of 345) after major thoracic surgery without perioperative VTE prophylaxis and the incidence of VTE after lung cancer surgeries was as high as 16.4% (40).
Interestingly, Agzarian et al. reported the VTE prevalence was 12.1% in patients undergoing oncologic lung resections, despite adherence to in-hospital VTE prophylaxis guidelines. The majority of VTE events in their group were asymptomatic, and all developed in the post-discharge time frame (37).
Current status of VTE prophylaxis in thoracic surgery in China
It has been reported that 96% of surgeons in the United States agreed that comprehensive prevention measures against VTE should be taken intra-operatively and postoperatively (41). However, the prevention against VTE has not been widely implemented in China. VTE prophylaxis is practiced differently in many hospitals in China. Based on robust evidence, consensus guidelines from multiple specialty organizations provide clear recommendations for the prevention of VTE, and highlight the need for prophylaxis in cancer patients (4,5,16,18,19,23,27,42). Despite this, VTE prophylaxis remains underused in cancer patients in China. According to a national survey, 35.35% (407/1,150) of the Chinese thoracic surgeons did not use any thromboprophylaxis after lung cancer surgery (43). Possible explanations may be: (I) most Chinese surgeons believe that the incidence of VTE is low; (II) concerns about the possibility of major bleeding after chemoprophylaxis; and (III) concerns about the increase of medical costs (any benefits of anticoagulation may not be demonstrated in a short term due to high cost of PE treatment). Thus, awareness of the prevention and treatment of VTE should be increased among clinicians, especially thoracic surgeons in China.
Risk factors for VTE and their assessment and stratification
Risk factors
Many of the risk factors for development of VTE are common to patients with cancer. In principle, all causes of vein injuries, stagnation of venous blood flow, blood hyper coagulation, malignancies, chemotherapy administration, and cancer surgery are recognized risk factors of VTE. The factors related to the development of VTE in cancer patients can be patient related (e.g., age, BMI), cancer related (e.g., histopathologic type of cancer), treatment related (e.g., surgical procedure, chemotherapy), or a combination of these factors (Corrales-Rodriguez L, Blais N. Lung cancer associated venous thromboembolic disease: a comprehensive review. Lung Cancer 2012;75:1-8.) (5,18).
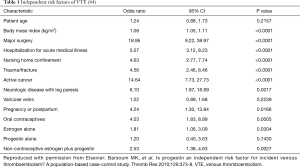
It is important to identify patients who are at a high risk of experiencing a VTE following lung cancer surgery. The preoperative risk factors of VTE include prior history of VTE, surgery, trauma, bed rest, malignant tumors and their treatment (hormones, chemotherapy, or radiotherapy), old age, underlying medical diseases (respiratory disease, myocardial infarction, congestive heart failure, ischemic stroke, pancreatitis, renal failure, and cardiac/respiratory failure caused by various reasons), obesity, smoking, varicose veins, and hereditary or acquired predisposition to thrombosis. Postoperative risk factors include immobilization, bedridden state, central venous accesses catheters and other intrusive devices, sepsis, use of sedative/anesthetic drugs, prolonged surgical time, and mechanical ventilation. These preoperative and postoperative risk factors often co-exist (5). Procedure-related risk factors should be also considered when assessing an individual’s risk of developing VTE. Procedure-related risk factors include duration of the procedure, degree of tissue damage, degree of immobility after surgery, type of the surgical procedure (e.g., wedge resection, lobectomy, pneumonectomy, sleeve resection, etc.) (14) (Table 1).
Lung cancer has been shown to be an independent risk factor for VTE (45), and the disease may have numerous mechanisms that predisposes a patient to having VTE, including chemotherapy treatment. Primary bronchogenic carcinoma, especially adenocarcinoma, can damage the balance between the deposition and degradation of fibrin in vascular system. Patients with lung cancer or esophageal cancer often have coagulation anomalies. Increased fibrin degradation products, thrombocytosis, hyperfibrinogenemia, and prolonged prothrombin time, are suggestive of the presence of chronic disseminated intravascular coagulation. Tumor tissues can secrete coagulants such as tissue factors, platelet aggregation-promoting substances, polysaccharides, and plasmin activators, which can induce hypercoagulable state and thus promote a predisposition to thrombosis. Vascular intima that has been infiltrated by cancer cells loses its antithrombotic ability. As a result, the solid tumor itself can potentially cause thrombosis and neoplasms on vascular walls and cardiac valves.
Lung cancer surgery is a risk factor for VTE, and many acquired factors can further promote the occurrence of VTE (5). Peri-operative blood loss and tissue injury can further activate the coagulation system and thus increase the risk of VTE. Postoperative confined to bed can lead to blood stasis. Hemostasis drugs may be applied because of concerns about intraoperative bleeding or due to the presence of bleeding. Surgical stress and potential organ dysfunction may also trigger a predisposition to thrombosis. Hematoma compression, prolonged operating time, trauma/sepsis secondary to trauma, placement of a central venous catheter, and other factors, can all cause vascular endothelial injury. This can lead to a decrease in venous pressure, diminished flow rate and vascular stenosis, all of which can further activate the coagulation system and promote the formation of blood clots. During the surgery itself, limb hypo perfusion and decreased venous blood flow due to anesthesia are both risk factors for lower extremity DVT.
The incidence of VTE vary substantially between cancer patients, considerably depending on clinical factors, the most important being tumor type and stage (14,39). Ziomel et al. found that primary bronchogenic carcinoma (25%), adenocarcinoma (44%), cancer sized 3 cm or larger (47%), stage II lung cancer (50%) and pneumonectomy or lobectomy (29%) have been associated with a higher risk of VTE than metastatic tumors and benign tumors (0%), other lung cancer types (12%), small cell lung cancer (15%), stage I lung cancer (21%), and segmentectomy or wedge resection (4%) (39).
Risk assessment and stratification
Although it is commonly stated that cancer patients are at high risk for VTE, there is significant variation in risk amongst subgroups of this population. Discriminating between low- and high-risk patients is therefore crucial to optimize the risk-benefit ratio of thromboprophylaxis.
A variety of methods can be applied for evaluating the risk factors of VTE. Available VTE risk scoring models include Caprini risk assessment model (Caprini RAM) (46), Rogers score (47), Padua prediction score (48), Wells score (49), and Khorana score (45). All these are tailored tool for assessing the risks of VTE.
Both Caprini RAM and Rogers score are recommended by the American College of Chest Physicians (ACCP) guidelines for risk assessment in surgical patients (19), while Padua score and Wells score are considered the most suitable risk assessment models for in-hospital medical patients (42,50) and the Khorana score is for cancer patients receiving chemotherapy (45).
The studies have demonstrated that the Caprini RAM has been widely applied in thoracic surgery (46,51-53).
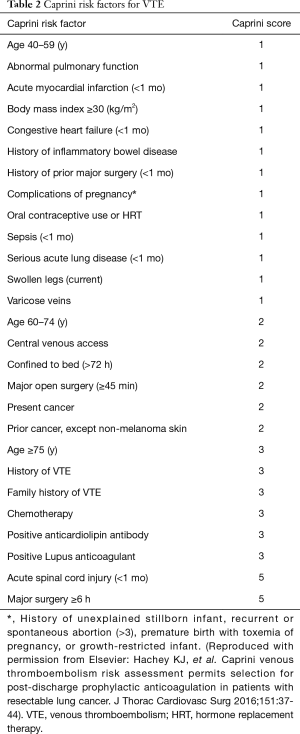
The classic Caprini scale includes about 40 risk factors (including congenital and/or acquired risk factors), covering almost all the risk factors for VTE in a hospitalized patient. Each risk factor is assigned with a different score based on the risk level (1,46,51-53). The patients are then further rated as “low risk”, “intermediate risk”, “high risk”, and “very high risk”, and corresponding prevention measures are recommended. The validity of the Caprini scale has been well documented in Western countries (51-53). Different prevention measures for VTE are recommended based on the risk levels. Thus, the scale not only enables the prediction of the risk for VTE but also recommends corresponding prevention measures, including the type and duration of a specific prevention measure.
Recently, a modified Caprini scale has been used which is tailored for the risk factors following thoracic surgery (53,54). As shown in Table 2, the modified Caprini scale simplifies the risk into three levels: 0–4, low risk; 5–8, intermediate risk; and ≥9, high risk.
Full table
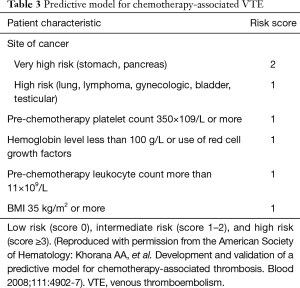
The Khorana score was developed as a model for predicting the risk of VTE in patients on chemotherapy, to identify which high-risk patients would benefit from prophylactic anticoagulation. As shown in Table 3, the Khorana score includes variable such as tumor location, platelet count, hemoglobin levels, white blood cell count, and BMI (45). The Khorana score has been externally validated. However, this score did not perform well on patients with lung cancer as shown in a study by Mansfield et al. (55).
Full table
Clinical characteristics and diagnosis of VTE
Clinical characteristics of VTE
Even minimal postoperative DVT may induce fatal PE in patients with pulmonary carcinoma. Before the fatal outcome of PE, some mild symptoms including dyspnea and chest pain may be observed and are often misdiagnosed as cardiac failure, sudden cardiac death, lung atelectasis or pulmonary infection. Moreover, underlying thoracic conditions and postoperative symptoms commonly masks the signs and symptoms of PE. Thus, it is important to increase the awareness for PE.
The typical clinical manifestations of PE include dyspnea, chest pain, hemoptysis and/or circulatory collapse, the so-called triple signs of PE; however, the occurrence of all these manifestations is rarely seen in clinical practice. For patients who have undergone thoracic surgery, any minimal suspected manifestation of embolism should be closely monitored and evaluated.
PE should be highly suspected if any of the following manifestations is observed after the surgery:
- Progressive aggravation of hypoxemia and hypocapnia during spontaneous breathing;
- Further exacerbation of hypoxemia in patients receiving controlled ventilation under sedation (without other identifiable cause);
- Patients with chronic pulmonary lesions and known CO2 retention experience dyspnoea and worsening hypoxaemia, along with decreased PaCO2;
- Fever of unknown origin;
- Sudden increase of pulmonary arterial pressure and central venous pressure during hemodynamic monitoring.
Progressive aggravation of hypoxemia and hypocapnia during spontaneous breathing;
Further exacerbation of hypoxemia in patients receiving controlled ventilation under sedation (without other identifiable cause);
Patients with chronic pulmonary lesions and known CO2 retention experience dyspnoea and worsening hypoxaemia, along with decreased PaCO2;
Fever of unknown origin;
Sudden increase of pulmonary arterial pressure and central venous pressure during hemodynamic monitoring.
Diagnosis of VTE
Due to difficulties in postoperative transfer, bedside Doppler ultrasonography for lower extremities and echocardiography (both transesophageal or transthoracic) are often used as the preferred initial examinations for patients with suspected diagnosis. Doppler ultrasonography can diagnose the presence of any DVT. Transesophageal echocardiography can detect changes in right heart functions, indirectly suggesting the presence of PE. Transesophageal echocardiography may also identify the presence of a thromboembolism in the main pulmonary artery.
D-dimer can be used as an exclusion criterion of acute DVT-PE for non-surgical patients; however, since the coagulation-fibrinolysis system is activated by the surgery itself and by underlying pathological changes and the preexisting malignancy, the D-dimer may be a priori elevated and thus its diagnostic value, when evaluated, is limited. Therefore, in clinical practice D-dimer testing is not recommended for the diagnosis of VTE in cancer patients. However, a negative finding of D-dimer is considered to be highly valuable for exclusion of VTE (5).
Ventilation/perfusion scintigraphy had previously been used as the preferred examination. PE can be ruled out directly based on low-probability or negative scintigraphic findings, and a diagnosis of PE can be made based on highly suspected findings (5). As for moderately suspected patients, pulmonary angiography may be advised; however, this may extend the time to a confirmed diagnosis and even delay the proper diagnosis and treatment.
In recent years, the spiral CTPA method has been increasingly applied and considered as the gold standard for diagnosis (5). It has a specificity of >95% in diagnosing PE, and a very high negative predictive value. CTPA has been widely accepted as the preferred examination for suspected non-massive and acute PE and should be completed within 24 hours of initiation of symptoms.
Although venography and pulmonary angiography have been considered as the gold standard for the diagnoses of DVT and PE, the cardiopulmonary reserve capacity should also be adequately evaluated. The appropriate diagnostic modalities should be carefully selected after balancing the advantages and disadvantages. However, as for patients with clinically highly suspected diagnosis of massive PE, concomitant with contraindications for thrombolytic agents or anticoagulants, pulmonary angiography can be implemented directly and preparations for interventions should be made, which may substantially reduce the time and cost required for examinations.
Prevention of postoperative VTE
Principals of prophylaxis
The National Comprehensive Cancer Network (NCCN) guidelines (5), 9th ACCP guidelines (19), the American Society of Clinical Oncology (ASCO) guidelines (56), the European Society of Medical Oncology (ESMO) guidelines (21), the International Initiative on Thrombosis and Cancer (ITAC-CME) (4), all categorize hospitalized patients with active cancer as a group at high risk for VTE, and should be considered for pharmacological thromboprophylaxis throughout hospitalization, either preoperatively or as early as possible after the operation.
In general, prevention measures include basic nursing care, mechanical and pharmacological prophylaxis (18,57,58). Pharmacological thromboprophylaxis with unfractionated heparin (UFH) or low molecular weight heparin (LMWH) is the most important measure and can improve outcomes and reduce mortality rates in surgical patients, including cancer patients (5,10,19,21,59).
In patients with lung cancer, especially for those at high risk of VTE, proactive peri-operative prevention interventions are important to lower the incidence of VTE. The simplest way is to apply Caprini risk scale for classification. For lung cancer patients at low risk for VTE (scored 0–4), mobilization as early as possible, along with mechanical prophylaxis are recommended. For lung cancer patients at moderate risk (scored 5–8) who are not at high risk for major bleeding, low-dose unfractionated heparin (LDUH) or LMWH is recommended as anticoagulation therapy, or mechanical prophylaxis. For lung cancer patients at high risk for VTE (scored ≥9) who are at low or moderate risk for major bleeding, chemoprophylaxis, along with mechanical prophylaxis are recommended. For lung cancer patients at high risk for VTE (scored ≥9) who are also at high risk for major bleeding, mechanical prophylaxis is the best choice of prevention method, chemoprophylaxis should be added as soon as the risk of major bleeding diminished (see Table 4) (4,5,19,23,27,58,59).

Full table
Basic nursing care
The basic nursing care can play an important role in prevention of postoperative VTE.
Encourage patients to mobilise as soon as possible
Early mobilisation is recommended. Some studies have supported that early mobilisation may prevent stasis and reduces subsequent risk of thrombi formation (53).
Leg exercises
The most simple, safe and effective way of increasing venous return to the heart is leg exercises or leg elevation which can contribute to the prevention of thrombi formation (60). For patients with a severe condition, difficulty in mobilization, or requirement for a long-term bed rest, mechanical devices may use for continuous passive motion.
Avoid dehydration
It has been reported that perioperative dehydration is closely associated with VTE. Therefore, dehydration after surgery should be avoided unless clinically necessary (61).
Patients education
Patients with lung cancer should be educated about the significance of VTE. Advice the patients to improve their lifestyle: smoking and alcohol cessation, control of blood glucose or blood lipids, etc.
Mechanical prophylaxis
If the bleeding risk and thrombosis risk are both high, mechanical prophylaxis using intermittent pneumatic compression devices (IPC) is recommended (5). Combinations with chemoprophylaxis are recommended for patient at high risk for VTE with low-moderate risk of major bleeding. Mechanical prophylaxis is not recommended as monotherapy except for patients at low risk for VTE, or for patients with concomitant coagulation disorders and/or at high risk for major bleeding when pharmacological methods are contraindicated (see Table 4) (5,27,58,62).
Pharmacological prophylaxis
General principals of pharmacological prophylaxis
Treatment for preventing thromboembolism should be performed in all patients undergoing thoracic malignancy-related surgeries. ACCP guidelines on perioperative VTE prevention recommend preoperative usage of in-hospital thromboprophylaxis with either LDUH or LMWH (18,19). For high-risk patients, mechanical prophylaxis with sequential compression devices should be added. Similar recommendations were proposed by the British NICE (27). The use of LMWH or LDUH as VTE prophylaxis in cancer patients undergoing major cancer surgery is recommended by the ESMO guidelines (21).
Patients undergoing chest and/or abdominal surgery or procedure under minimally invasive or thoracotomy/laparotomy for more than 30 minutes should receive the postoperative treatment of LDUH or LMWH therapy, unless there is active bleeding or the patient is at high-risk of bleeding.
However, benefits of pharmacological prophylaxis should be weighed against the risk of bleeding as bleeding could lead to serious consequences. Therefore, pharmacological prophylaxis should be initiated after weighing the bleeding risk versus thrombotic risk (27).
Timing and duration of pharmacological prophylaxis
The timing of prophylaxis for surgical patients is a very important issue. Current data are available confirming the benefits of prophylactic anticoagulation during perioperative period. Anticoagulant therapy should be initiated preoperatively (2–12 hours prior to surgery) and should be given postoperatively within 6–12 hours (63).
For patients at moderate risk of VTE, postoperative prophylaxis of anticoagulation should continue for at least 7–14 days. For patients at a high risk (including those with postoperative residual tumor, obesity, or a prior history of VTE), the anticoagulation prophylaxis should be extended to four weeks after surgery (42,64-66).
The most recent 2016 ACCP10 guidelines on VTE prevention outline perioperative thromboprophylaxis regimens for selected surgical populations. For patients undergoing hip or knee replacement, there is grade 1B evidence for short duration (10–14 days) and grade 2B evidence for extended-duration thromboprophylaxis (32). This recommendation is based on large randomized controlled trials that have shown that extended post-operative prophylaxis (up to 42 days) is superior to in-hospital prophylaxis only for the prevention of both asymptomatic (screening venography detected) and symptomatic VTE (67-72). The ASCO guidelines recommend that all major cancer surgeries receive prophylaxis starting before surgery and continuing for at least 7–10 days, and extended prophylaxis recommended for up to 4 weeks total for major high-risk abdominal or pelvic surgery (19). However, there is no guidance from either the ACCP (19) nor the ASCO (42) as to the need for extended-duration prophylaxis in thoracic surgery patients.
Drug recommendations
The existing anticoagulant drugs in China include UFH, LMWH, fondaparinux, vitamin K antagonists (VKA) and NOACs. LMWH is generally considered to be the preferred anticoagulant for patients with cancer, and fondaparinux sodium or warfarin-based prophylaxis may be considered for patients who cannot tolerate LMWH or UFH. The characteristics and recommended regimen of several anticoagulants were described as follows:
UFH
Current data has confirmed that UFH was associated with reduction in the odds of DVT formation in lower extremities and reduction in the odds of PE, alone with increase in the odds of nonfatal major bleeding (57). To minimize the risk of major bleeding complication, 5,000 U UFH 3 times a day is recommended in clinical practice and routine hemostasis monitoring (activated partial prothrombin time, APPT) is required for adjusting dosage (5).
Recommended dose for UFH is presented in Table 5.

Full table
LMWH
LMWH has the following features:
- Easy to use, allowing dose adjustment based on body weight and subcutaneous injection;
- Relatively low incidence of severe bleeding complications with improved safety profile;
- Routine hemostasis monitoring is not required.
Use of LMWH once a day is recommended to prevent postoperative VTE in lung cancer patients. Pharmacological prophylaxis should be started 2–12 h preoperatively depending on different type of LMWH agents, and continued for at least 5–10 days. There is no strong evidence allowing conclusions regarding the superiority of one type of LMWH over another.
Recommended doses for LMWH are presented in Table 5.
Xa factor inhibitors
Xa factor inhibitors are characterized by a wide therapeutic window and fixed doses. No routine hemostasis monitoring is required during Xa factor inhibitor treatment. These drugs can be applied for heparin-induced thrombocytopenia. Compared with LMWH, Xa factor inhibitors can significantly reduce the incidence of phlebothrombosis without increasing the risk of bleeding. However, there is no evidence to support fondaparinux as an alternative to LMWH for the prophylaxis of postoperative VTE in cancer patients (4).
Recommended doses for Xa factor inhibitors are presented in Table 5.
VKA
VKA (e.g., warfarin) is an option for long-term treatment of VTE in cancer patients (5). However, VKA have some significant shortcomings including: (i) narrow therapeutic window, with high individual variation; routine INR monitoring is required to maintain the target INR of 2.0–3.0 by dose adjustment; INR >3.0 may increase the risk of hemorrhage; and (ii) numerous medications and dietary factors interact with warfarin and can increase or decrease anticoagulation effect of warfarin. Therefore, VKA is not recommended for surgical patients.
NOACs
There are currently few data of phase III trials on the safety and efficacy in lung cancer of the NOACs. Use of NOACs for prevention of VTE in patients with malignancy is not currently recommended (58).
Contraindications and precautions for thromboprophylaxis
Contraindications for thromboprophylaxis can be divided into two categories: relative or absolute.
- Absolute contraindications: (a) recent active bleeding and coagulation disorders; (b) compartment syndrome; (c) severe cranial trauma or acute spinal cord injury; (d) platelet count less than 20×109/L; and (e) heparin and LMWH are contraindicated in patients with heparin-induced thrombocytopenia (5);
- Relative contraindications: (a) prior history of intracranial hemorrhage; (b) prior history of GI hemorrhage; (c) acute intracranial injury or mass; (d) platelet count falling to [20–100]×109/L; and (e) patients with rheumatoid retinopathy (5);
- Precautions: (a) due to differences in mechanism of action, molecular weight, unit, dose and activities of anti-Xa and anti-IIa factors, only one drug can be used during the prophylaxis. Substitution by another drug is not allowed. All instructions for precautions and adverse reactions should be followed for each drug; (b) for patients with renal or hepatic impairment, the dosage should be adjusted with caution. LMWH, Fondaparinux sodium and Rivaroxaban should not be used in patients with severe renal impairment; (iv) Since major bleeding is the most serious complication for medication, risk factors of major hemorrhage should be assessed pre-operatively (19) (Table 6).

Full table
Inferior vena cava filter
Inferior vena cava filters are not recommended for primary VTE prophylaxis in cancer patients. Absolute contraindication for mechanical and pharmacological VTE prophylaxis or complications from anticoagulation are the principal indication for inferior vena cava filter placement and retrievable filter is recommended (5,27).
Summary
Lung cancer patients are at high risk for developing thrombosis and recurrent VTE complications following lung cancer surgery. There are various risk factors related to the development of VTE. An individual risk assessment model is helpful to further stratify the risk and recommend appropriate prophylaxis. Effective thromboprophylaxis reduces the risk for VTE and improves outcomes. Thromboprophylaxis should be mandatory part of perioperative care. Pharmacological prophylaxis does not lead to increased bleeding risk. There is relatively small amount of research and previous experience in the lung resection population, so far the guidelines were extrapolated based on other surgical specialties. However, there is an emerging interest with some recent publications, and it is therefore hoped that, with better collaboration and research, robust local evidence becomes available, more thoracic surgery guidelines could be developed.
Acknowledgements
The authors would like to thank the secretaries Grace S. Li (Science Editor, The Society for Translational Medicine) and Maxine Y. Feng (Science Editor, The Society for Translational Medicine) for their help and comments on this article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dentali F, Malato A, Ageno W, et al. Incidence of venous thromboembolism in patients undergoing thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2008;135:705-6. [Crossref] [PubMed]
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285-91. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Farge D, Bounameaux H, Brenner B, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2016;17:e452-66. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Venous Thromboembolic Disease Clinical Practice Guidelines in Oncology. Version 1.2017. Available online: http://www.nccn.org/professionals/default.aspx
- Rickles FR. Thrombosis and lung cancer. Am Rev Respir Dis 1989;140:573-5. [Crossref] [PubMed]
- Gabazza EC, Taguchi O, Yamakami T, et al. Evaluating prethrombotic state in lung cancer using molecular markers. Chest 1993;103:196-200. [Crossref] [PubMed]
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. [Crossref] [PubMed]
- Shinagare AB, Okajima Y, Oxnard GR, et al. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer 2012;78:161-6. [Crossref] [PubMed]
- Khorana AA. The NCCN Clinical Practice Guidelines on Venous Thromboembolic Disease: strategies for improving VTE prophylaxis in hospitalized cancer patients. Oncologist 2007;12:1361-70. [Crossref] [PubMed]
- Hicks LK, Cheung MC, Ding K, et al. Venous thromboembolism and nonsmall cell lung cancer: a pooled analysis of National Cancer Institute of Canada Clinical Trials Group trials. Cancer 2009;115:5516-25. [Crossref] [PubMed]
- Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost 2008;6:601-8. [Crossref] [PubMed]
- Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458-64. [Crossref] [PubMed]
- Vitale C, D'Amato M, Calabrò P, et al. Venous thromboembolism and lung cancer: a review. Multidiscip Respir Med 2015;10:28. [Crossref] [PubMed]
- Mandalà M, Falanga A, Piccioli A, et al. Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM). Crit Rev Oncol Hematol 2006;59:194-204. [Crossref] [PubMed]
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490-505. [Crossref] [PubMed]
- Farge D, Bosquet L, Kassab-Chahmi D, et al. 2008 French national guidelines for the treatment of venous thromboembolism in patients with cancer: report from the working group. Crit Rev Oncol Hematol 2010;73:31-46. [Crossref] [PubMed]
- Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism--International Consensus Statement. Int Angiol 2013;32:111-260. [PubMed]
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-77S. Erratum in: Chest 2012;141:1369.
- Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22:414-23. [Crossref] [PubMed]
- Mandala M, Falanga A, Roila F, et al. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22:vi85-92. [Crossref] [PubMed]
- Debourdeau P, Kassab Chahmi D, Le Gal G, et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol 2009;20:1459-71. [Crossref] [PubMed]
- Afshari A, Ageno W, Ahmed A, et al. European Guidelines on perioperative venous thromboembolism prophylaxis: Executive summary. Eur J Anaesthesiol 2018;35:77-83. [PubMed]
- Al-Hameed F, Al-Dorzi HM, AlMomen A, et al. Prophylaxis and treatment of venous thromboembolism in patients with cancer: the Saudi clinical practice guideline. Ann Saudi Med 2015;35:95-106. [Crossref] [PubMed]
- Easaw JC, Shea-Budgell MA, Wu CM, et al. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 1: prophylaxis. Curr Oncol 2015;22:133-43. [Crossref] [PubMed]
- Khorana AA, Carrier M, Garcia DA, et al. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:81-91. [Crossref] [PubMed]
- National Institute for Health and Care Excellence guidelines (National Institute for Health and Care Excellence. Venous thromboembolism: reducing the risk for patients in hospital, Clinical Guideline CG92. Updated June 2015. Available online: https://www.nice.org.uk/guidance/cg92/chapter/1-recommendations
- Trinh VQ, Karakiewicz PI, Sammon J, et al. Venous thromboembolism after major cancer surgery: temporal trends and patterns of care. JAMA Surg 2014;149:43-9. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol 2006;24:484-90. [Crossref] [PubMed]
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007;14:929-36. [Crossref] [PubMed]
- Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013;119:648-55. [Crossref] [PubMed]
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484-8. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632-4. [Crossref] [PubMed]
- Tagalakis V, Levi D, Agulnik JS, et al. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol 2007;2:729-34. [Crossref] [PubMed]
- Zhang Y, Yang Y, Chen W, et al. Prevalence and associations of VTE in patients with newly diagnosed lung cancer. Chest 2014;146:650-8. [Crossref] [PubMed]
- Connolly GC, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer 2012;78:253-8. [Crossref] [PubMed]
- Agzarian J, Hanna WC, Schneider L, et al. Postdischarge venous thromboembolic complications following pulmonary oncologic resection: An underdetected problem. J Thorac Cardiovasc Surg 2016;151:992-9. [Crossref] [PubMed]
- Chen W, Zhang Y, Yang Y, et al. Prognostic significance of arterial and venous thrombosis in resected specimens for non-small cell lung cancer. Thromb Res 2015;136:451-5. [Crossref] [PubMed]
- Ziomek S, Read RC, Tobler HG, et al. Thromboembolism in patients undergoing thoracotomy. Ann Thorac Surg 1993;56:223-6; discussion 227. [Crossref] [PubMed]
- Song CF, Li H, Tian B, et al. Incidence of postoperative venous thromboembolism after thoracic surgery and its characteristic: a single center, prospective cohort study. Zhonghua Wai Ke Za Zhi 2018;56:284-8. [PubMed]
- Caprini JA, Arcelus J, Sehgal LR, et al. The use of low molecular weight heparins for the prevention of postoperative venous thromboembolism in general surgery. A survey of practice in the United States. Int Angiol 2002;21:78-85. [PubMed]
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e195S-226S.
- Song CF, Li H, Tian B, et al. Survey of current status of prevention of venous thromboembolism after thoracic surgery in China. Zhonghua Wai Ke Za Zhi 2017;55:661-6. [PubMed]
- Barsoum MK, Heit JA, Ashrani AA, et al. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res 2010;126:373-8. [Crossref] [PubMed]
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902-7. [Crossref] [PubMed]
- Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol 2001;38:12-9. [Crossref] [PubMed]
- Rogers SO Jr, Kilaru RK, Hosokawa P, et al. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1211-21. [Crossref] [PubMed]
- Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 2010;8:2450-7. [Crossref] [PubMed]
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795-8. [Crossref] [PubMed]
- Qaseem A, Snow V, Barry P, et al. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2007;146:454-8. [Crossref] [PubMed]
- Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon 2005;51:70-8. [Crossref] [PubMed]
- Caprini JA. Individual risk assessment is the best strategy for thromboembolic prophylaxis. Dis Mon 2010;56:552-9. [Crossref] [PubMed]
- Hachey KJ, Hewes PD, Porter LP, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg 2016;151:37-44.e1. [Crossref] [PubMed]
- Hachey KJ, Sterbling H, Choi DS, et al. Prevention of Postoperative Venous Thromboembolism in Thoracic Surgical Patients: Implementation and Evaluation of a Caprini Risk Assessment Protocol. J Am Coll Surg 2016;222:1019-27. [Crossref] [PubMed]
- Mansfield AS, Tafur AJ, Wang CE, et al. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost 2016;14:1773-8. [Crossref] [PubMed]
- Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2189-204. [Crossref] [PubMed]
- Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654-6. [Crossref] [PubMed]
- Ahmed AB, Koster A, Lance M, et al. European guidelines on perioperative venous thromboembolism prophylaxis: Cardiovascular and thoracic surgery. Eur J Anaesthesiol 2018;35:84-9. [PubMed]
- Kierkegaard A, Norgren L, Olsson CG, et al. Incidence of deep vein thrombosis in bedridden non-surgical patients. Acta Med Scand 1987;222:409-14. [Crossref] [PubMed]
- Rosengarten DS, Laird J. The effect of leg elevation on the incidence of deep-vein thrombosis after operation. Br J Surg 1971;58:182-4. [Crossref] [PubMed]
- Kelly J, Hunt BJ, Lewis RR, et al. Dehydration and venous thromboembolism after acute stroke. QJM 2004;97:293-6. [Crossref] [PubMed]
- Afshari A, Fenger-Eriksen C, Monreal M, et al. European guidelines on perioperative venous thromboembolism prophylaxis: Mechanical prophylaxis. Eur J Anaesthesiol 2018;35:112-5. [PubMed]
- Liew NC, Alemany GV, Angchaisuksiri P, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol 2017;36:1-20. [PubMed]
- Jacobson BF, Louw S, Büller H, et al. Venous thromboembolism: prophylactic and therapeutic practice guideline. S Afr Med J 2013;103:261-7. [Crossref] [PubMed]
- Kakkar VV, Balibrea JL, Martínez-González J, et al. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost 2010;8:1223-9. [Crossref] [PubMed]
- Rasmussen MS, Jorgensen LN, Wille-Jørgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost 2006;4:2384-90. [Crossref] [PubMed]
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975-80. [Crossref] [PubMed]
- Planes A, Vochelle N, Darmon JY, et al. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: double-blind randomised comparison of enoxaparin versus placebo. Lancet 1996;348:224-8. [Crossref] [PubMed]
- Dahl OE, Andreassen G, Aspelin T, et al. Prolonged thromboprophylaxis following hip replacement surgery--results of a double-blind, prospective, randomised, placebo-controlled study with dalteparin (Fragmin). Thromb Haemost 1997;77:26-31. [PubMed]
- Lassen MR, Borris LC, Anderson BS, et al. Efficacy and safety of prolonged thromboprophylaxis with a low molecular weight heparin (dalteparin) after total hip arthroplasty--the Danish Prolonged Prophylaxis (DaPP) Study. Thromb Res 1998;89:281-7. [Crossref] [PubMed]
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin extended out-of-hospital vs in-hospital warfarin/out-of-hospital placebo in hip arthroplasty patients: a double-blind, randomized comparison. North American Fragmin Trial Investigators. Arch Intern Med 2000;160:2208-15. [Crossref] [PubMed]
- Comp PC, Spiro TE, Friedman RJ, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. J Bone Joint Surg Am 2001;83-A:336-45. [Crossref] [PubMed]