Pancoast tumors: characteristics and preoperative assessment
Introduction
Superior sulcus tumors (SSTs), or as otherwise known Pancoast tumors, make up a clinically unique and challenging subset of non-small cell carcinoma of the lung (NSCLC). Although the outcome of patients with this disease has traditionally been poor, recent developments have contributed to a significant improvement in prognosis of SST patients. SSTs are characterized by the infiltration of the thoracic inlet and may present with a constellation of symptoms depending on the invasion of specific structures. The combination of severe and unrelenting shoulder and arm pain along the distribution of the eighth cervical and first and second thoracic nerve trunks, Horner’s syndrome (ptosis, miosis, and anhidrosis) and atrophy of the intrinsic hand muscles comprises a clinical entity named as “Pancoast-Tobias syndrome”. Apart NSCLC, other lesions may, although less frequently, result in Pancoast syndrome.
Definition, history, epidemiology and anatomic features of Pancoast tumors
Definition
The name SSTs is associated with an anatomical groove present in the lung apex, formed by the subclavian artery as it passes over the lung at this point. Not all SSTs are associated with this precise anatomic location, but the term has come to signify any tumor that is identified in the apices of the lungs with the associated clinical symptoms that are the hallmark of this disease. These tumors are often referred to as Pancoast tumors, after the radiologist Henry Pancoast described these lesions, developing at the chest apex, as carcinomas of uncertain origin in 1924 and 1932 (1,2).
The following criteria must be met for characterizing a lesion as a Pancoast tumor; the tumor must invade the parietal pleura and cause pain, paresthesias or other neurological dysfunction; it is not sufficient only to have an apical lung tumor. These tumors may invade muscles, upper ribs, thoracic vertebral bodies, subclavian vessels, the inferior portion of the brachial plexus, and the upper end of the thoracic autonomic chain including the stellate ganglion. Invasion of the brachial plexus leads to the constellation of neurologic signs and symptoms known as the Pancoast syndrome, whereas destruction of the stellate ganglion by tumor causes Homer syndrome.
Historical background
The first recorded case of a Pancoast tumor was described by Hare in 1838 (3). This “tumor involving certain nerves”, was producing constant and characteristic pain in the shoulder and the arm. Almost ninety years later in 1924, Henry K. Pancoast, a radiologist from Philadelphia, reported several cases of chest tumors associated with characteristic radiographic findings of “small homogenous shadows at the extreme apex”, “more or less” rib destruction and often vertebral infiltration (1). These tumors were associated with a clinical syndrome of pain in the distribution of the eighth cervical and first and second thoracic trunks and Horner’s syndrome. Eight years later he published a second report (2) on his original work upon the syndrome, reporting on seven such patients. He stated that these tumors were “not subject to surgical removal”, were “refractory to radiation treatment” and were “rather rapidly fatal”.
In 1946, Herbert and Watson (4) reviewed all published cases until then and presented eight new cases of Pancoast tumors. They concluded that the disease without effective therapy was uniformly fatal. All patients that they observed died within ten months after initial diagnosis was made. It was not until 1954 that Haas and colleagues (5) described palliation of the severe and unrelenting arm pain troubling these patients; they reported dramatic pain relief after external beam radiation. One of those patients survived for almost three years after initiation of therapy.
Chardack and MacCallum (6) were the first to achieve long term survival (>5 years) by means of combination therapy consisting in lobectomy followed by external beam radiation. This report was the basis for others to follow, suggesting that combined therapy might be appropriate for these patients. In 1961, Shaw and Paulson (7) presented a series of patients who were treated with radiotherapy preoperatively followed by surgical resection. However, these initial attempts at surgical resection were not widely accepted; reports of significant numbers of patients who received palliative therapy alone without surgery continued to appear in the literature (8). Nevertheless, combination therapy remained the mainstay of therapy for Pancoast tumors for over three decades. At the late 1990’s the introduction of concurrent chemo- and radio-therapy led to significant improvement in the outcome of the disease.
Epidemiology
Pancoast tumors represent 3% to 5% of all lung cancers, and are biologically similar to typical NSCLC with a predilection for distant metastasis (9). The major risk factor responsible for their development is cigarette smoking. The average age at presentation is the sixth decade of life, with men affected more frequently than women.
The most common cause of Pancoast syndrome is NSCLC of squamous cell origin (10) followed by adenocarcinoma and large cell carcinoma subtypes (11). However, in some studies adenocarcinoma has been reported to be more frequent than squamous cell subtype and since its incidence nowadays predominates in the developed countries, it may even overtake squamous cell carcinoma (12,13). The reasons underlying this shift have not been completely understood yet, but consumption of filtered cigarettes over the last decades has been highly incriminated. Small cell lung cancer is an infrequent cause of Pancoast tumor (14,15). Other primary apical neoplasms can also produce Pancoast syndrome; adenoid cystic carcinoma (16), carcinoid (17), hemangiopericytoma (18) and mesothelioma (19) have also been reported. Metastases to the lung from the larynx (19), thyroid (20), bladder (21) and cervix (22) have also been described. Hematologic malignancies such as plasmacytoma (23), non-Hodgkin lymphoma (24), and lymphomatoid granulomatosis (25) have also be listed as infrequent causes of Pancoast tumors. Finally pseudomonal (26) and staphylococcal (27) infections, as well pulmonary actinomycosis (28), have also been incriminated in the pathogenesis of a Pancoast-like syndrome (29). Rarely, tuberculosis (30), aspergillosis (31), cryptococcosis (32) and allescheriasis (33) have also been reported as possible causes.
Anatomic features of superior pulmonary sulcus tumors
In 1932 Pancoast reported that these tumors arose in a residual fissure formed during the period of embryonic development of the right upper lobe and the migration of the azygos vein; he stated that the original cells were likely epithelial rests from the fifth brachial cleft (2). On the contrary, Tobias suggested that the site of origin of these tumors was “bronchial pulmonary tissue” (34).
Variations exist among efforts made to describe the superior pulmonary sulcus as a definite anatomic structure. These contradictions can be explained because there can be both a functional definition of these tumors based on the characteristic presentation of the Pancoast syndrome, as well as an anatomic one based on the location of these lesions in the upper thoracic cavity.
Even nowadays the precise definition of the anatomic space of the superior sulcus remains inaccurate since most anatomy textbooks do not include its description as a defined anatomic area. In the original description, Pancoast described the tumor location as within the “superior pulmonary sulcus”, which later became well known as the “superior sulcus”. The superior sulcus therefore is considered the most cephalad extent of the chest wall, particularly the apical costovertebral gutter. In Kubic’s Surgical Anatomy of the Thorax (35) it is described as the “backward curve of the ribs which produces a deep groove internally on either side of the vertebral column”.
The term “superior sulcus” is obscured even more by those considering it to be within the lung itself and formed by the subclavian artery as it crosses the pulmonary apex (36). Paulson (37) agreed with this belief suggesting that most apical lung neoplasms arose in close proximity to this sulcus. On the other hand, Seydel and colleagues (38) suggested that SSTs arise in the fissure formed by the migration of the azygos vein during the development of the right upper lobe; if this were true, left-sided Pancoast tumors would not exist.
Netter (39) supported that the superior pulmonary sulcus actually “does not correspond to any recognized anatomic location”. These tumors were considered as apical carcinomas that involve the parietal pleura, upper ribs, endothoracic fascia, brachial plexus, sympathetic chain, vertebral bodies, with the resultant clinical syndrome.
Exceptions exist among those who tried to define the superior sulcus as a single consistent groove in the lung. Tumors have been shown to reside in pulmonary impressions formed by the first rib, azygos vein, superior vena cava, esophagus and inferior vena cava on the right and by the first rib, aortic arch, and descending aorta on the left side (40). Apical cancers do not commonly originate from the subclavian artery or vein sulcus. If they did, invasion of those vessels would be far more frequent.
Thus, this inconsistency by many authors and the difficulty to agree in a standard term defining the superior pulmonary sulcus has led to the rejection of that term. An understanding of the symptoms that associate the invaded anatomical structures and their radiologic documentation is critical in therapeutic planning.
Presentation, diagnostic workout, biology and staging of Pancoast tumors
Presentation
Superior sulcus lesions of NSCLC origin account for less than 5% of all bronchogenic carcinomas (9,41). Because the thoracic inlet represents a narrow compartment, modest growth and direct extension produce characteristic symptoms. Therefore, symptoms such as cough, hemoptysis, and dyspnea are uncommon in the initial stages of the disease due to the peripheral location of these tumors (9,29). The same stands for hilar pulmonary infiltration and mediastinal structure invasion. On the other hand, involvement of supraclavicular lymph nodes is not unusual, and the physical findings are related to the local invasion of nervous, vascular and bony structures of the apex by the process producing the full blown Pancoast’s syndrome.
In the early stages, shoulder pain represents the most common symptom; common causes include invasion of the parietal pleura, upper ribs, brachial plexus, endothoracic fascia, or the adjacent vertebral bodies. The pain may radiate down the ipsilateral arm following the typical distribution of the ulnar nerve. Thus, clinical features depend upon the location and type of structures invaded at the thoracic inlet by the tumor (see Table 1).
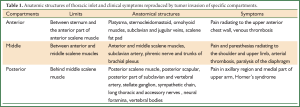
Full table
Tumors of the anterior compartment usually invade the first intercostal nerve and upper ribs rather than the phrenic nerve or superior vena cava, and usually present with pain distributed to the upper anterior chest wall.
Tumors of the middle compartment present with signs related to the compression or infiltration of the middle and lower trunks of the brachial plexus, such as pain and paresthesias irradiating to the shoulder and upper limb.
Tumors lying posterior to the middle scalene muscles present with a painful or abnormal sensation in the axilla and medial aspect of the upper arm in the territory of the intercostobrachial nerve.
Irritation of the sympathetic chain by the adjacent tumor can produce ipsilateral flushing and even hyperhidrosis of the face way before its invasion. Additionally reflex sympathetic dystrophy can also been reported. Further invasion of the sympathetic chain by the tumor results in Horner’s syndrome associated with ipsilateral ptosis, miosis, and anhidrosis, a phenomenon observed in 40% of patients (42). In only five percent of cases the tumor may involve the intervertebral foramina at the initial presentation (29). Diagnostic and radiologic work-up is always essential at presentation in order to assess surgical resectability and proceed to preoperative staging of the tumor.
Preoperative assessment
Upon presentation of a patient referring symptoms associated with the existence of a Pancoast tumor the physician should undergo a thorough and detailed preoperative work-up to establish thoracic inlet invasion. These steps should define the preoperative stage, histologically confirm the diagnosis and assess tumor’s resectability.
The keystone in the beginning of every preoperative workout should always be patient’s history and physical examination. After examining and understanding of the patient’s complains additional exams will be necessary. Pancoast tumors are not easily detected on plain chest X-rays (CXR) in their early stages of the disease, since they represent small apical tumors hidden behind the clavicle and the first rib. As the disease progresses, a CXR may reveal asymmetry of the pulmonary apices or pleural thickening; frequent suspicious findings include unilateral apical cap of more than 5 mm in thickness or asymmetries of both apical caps more than 5 mm, bone destruction or even thoracic wall and spinal invasion according to the stage. Lordotic views and radiographs of the cervical and upper thoracic spine may also be helpful in the determination of the presence of tumor (43,44).
CT scanning is an important diagnostic tool to define the size of the process, detect peripheral or satellite lesions and assess the presence of enlarged lymph nodes that are undetectable on conventional radiographs. CT scanning may also reveal bony, spinal, mediastinal or brachial plexus invasion (42). Enhancement with intravenous contrast medium injection can provide useful information about blood vessel involvement by the process.
Magnetic resonance imaging (MRI) of the chest is a more accurate preoperative examination in identifying the extent of the tumorous process than CT scan. It should be routinely performed when tumors approach the intervertebral foramina in order to rule out invasion of the extradural space. Additionally, MRI angiography offers a better assessment of invasion through the pleura and subpleural fat and the involvement of the subclavian vessels (45), brachial plexus (46), and vertebral bodies than CT scanning (43). CT scans provide 60% sensitivity, 65% specificity, and 63% accuracy in defining the local extent of tumor, in contrast to MRI with a sensitivity of 88%, a specificity of 100%, and an accuracy of 94%. Therefore MRI has evolved as the imaging modality of choice in the assessment of the local extent of Pancoast tumors.
Bronchoscopy with the flexible fiberoptic bronchoscope may assist in determining invasion of the tracheal or bronchial cavities and set diagnosis in about 30% to 40% of cases, since the majority of Pancoast tumors tend to grow in the periphery of the lung parenchyma (47). On the other hand, Narayan amd collegues (48) contradicted such a high percentage, suggesting that this is effective only up to 20% of cases.
Although 90% of all patients suffering from Pancoast tumor can be diagnosed in the basis of clinical and conventional radiologic methods alone, biopsy is mandatory for histological confirmation, operability assessment and therapy planning. Moreover, the wide variety of diseases that can result in Pancoast’s syndrome now mandates a conclusive diagnosis before definitive treatment is begun. The most sensitive method with a diagnostic yield of 95% is percutaneous transthoracic needle biopsy. This method can be performed through a posterior (with the patient prone) or cervical approach (inferior to brachial plexus and great vessels) with the use of ultrasonography, or CT scans for lesion localization (49,50). Additionally, video-assisted thoracoscopy (VAT) or even thoracotomy might be indicated for tissue diagnosis when other methods are inconclusive in order to eliminate the presence of local metastatic disease since patients with clinical N2 disease are not candidates for surgical excission.
Histological proof is also mandatory upon presence of mediastinal lymph node enlargement based on CXR or CT scanning. Because Pancoast lesions associated with mediastinal nodal metastases (positive N2 or N3 disease) have a poor prognosis, mediastinoscopy particularly in the right side, and/or anterior mediastinotomy may aid in defining the extend of the disease and should be strongly considered.
Over the last decade, positron emission tomography (PET) scan has gained significant popularity among preoperative work-up for lung cancer although its value remains questionable. In Pancoast tumors its role may be twofold. First it may be helpful in preoperative staging of lymph nodes (51) and detection of occult metastatic disease in those with NSCLC (52). Second, it can be used for restaging of tumors after neoadjuvant treatment (53). On the other hand, it does not provide useful topographic information about the primary lesion, unless there is associated atelectasis. Lymph nodes positive with PET scan require confirmation with mediastinoscopy, endobronchial ultrasound-guided biopsy or endoscopic ultrasound-guided biopsy. In addition, a negative PET-CT scan does not entirely exclude nodal involvement and a mediastinoscopy is still considered mandatory.
In cases of intrinsic hand muscle atrophy, neurologic examination is essential for assessment of nerve root involvement. Loss of T1 route is well tolerated, but removal of C8 route or lower trunk of the brachial plexus leads to loss of hand function and therefore are considered contraindication to surgery (54).
Finally, presence of distant metastases to other organs such as brain, liver and bones should be excluded preoperatively using computed tomography and radioactive bone scintigraphy.
If the tumor is considered resectable, routine preoperative work-up should be performed, in order to assess physical status, and register the patient as candidate for such a major procedure. Cardiopulmonary functional tests such as cardiologic evaluation, heart ultrasound, arterial blood gases and spirometry should be routinely performed before any major lung resection. Moreover, patient’s performance status and renal and neurological function must be adequate for platinum-based chemotherapy.
Tumor biology
Less than 50% of patients with Pancoast tumors are considered to have resectable lesions at initial presentation. The remaining percentage is unresectable because of extensive vertebral body involvement, mediastinal nodal disease, or distant metastases. This is in consistency with the overall trend of patients suffering from NSCLC. Additional observations suggest that the biology of Pancoast tumors does not qualitatively differ from NSCLC in general. It was originally proposed that Pancoast tumors had a low propensity to lymphatic or hematogenous spread and invasion was mainly due to local extension to adjacent structures (7). This impression is not valid today since pathologic nodal involvement (pN2 disease) is consistently found in 10% to 20% (55) of those initially considered not to exhibit any nodal involvement. Moreover, this finding is similar to the incidence of pN2 disease in other clinical stages of peripheral NSCLC (56). Additional evidence suggesting that the biology of Pancoast tumors is indifferent than that of other NSCLC, includes an increased survival when resection of a Pancoast tumor involves a lobectomy rather than a wedge resection alone. Lobectomy was associated with better overall survival than was incomplete pulmonary resection, and addition of intraoperative brachytherapy to resection did not seem to enhance survival (41).
Detterbeck (57) argued that the biology of Pancoast tumours is no different from that of NSCLC in general and that the unique character of Pancoast tumours seems not to lie in the tumour biology, but rather in the anatomy of the lung apex. Because these tumors by definition involve the chest wall, it is logical that these patients usually present with local rather than systemic manifestations of the disease. Furthermore, local extension of Pancoast tumors involves structures that are difficult to dissect technically and limit the extent of resection if major long term disability is to be avoided. Therefore, sometimes it is impossible to accomplish a R0 resection. Thus, the technical aspects of the anatomy of this region represent the most prominent unique feature of Pancoast tumors (57).
Staging
On the basis of T status, Pancoast tumors are staged at least as T3 due to invasion of the chest wall (see Table 2). Additional invasion of the vertebral body or the subclavian vessels upgrades the stage to T4. Ginsberg et al. (41) reported a 5-year survival less than 10% in those patients with vertebral body invasion. Additional studies support this concept associated with vertebral body invasion. Furthermore, tumors invading the subclavian vessels are also staged as T4. Dartevelle et al. (58) reported a 30% 5-year survival in T4 patients. However, subclavian vessel involvement was a negative prognostic factor.

Full table
The poor 5-year survival of Pancoast patients with pN2 or pN3 involvement underlines the importance of identifying these patients preoperatively. Thus, lymph node status is a very important prognostic factor. Since positive mediastinal N2 lymph nodes occur in about 20% of Pancoast tumors (59) the incidence of unsuspected N2 disease suggests further assessment of the mediastinum by means of mediastinoscopy as previously mentioned or PET, even in those patients without radiological sings of lymph node infiltration. Although in the past, mediastinoscopy was not routinely performed in most studies, Paulson (60) underlined the importance of mediastinoscopy as a preoperative staging method, since SST patients exhibited poor prognosis if mediastinal or hilar lymph nodes were involved.
Invasion of ipsilateral supraclavicular lymph nodes by the tumor is classified as N3 disease. Some series show that patients with supraclavicular lymph node metastases had a better prognosis than patients with N2 disease. Evidence suggests that such involvement in patients with a Pancoast tumor may not prevent long-term survival since may have a prognostic significance similar to that of N1 disease. These nodes are in close vicinity of the tumor and therefore could have the characteristics of the biological behavior of local nodes. Ginsberg reported a 5-year survival of 14% in patients with N3 disease as opposed to 0% in patients with N2 disease (41). Nevertheless, with the introduction of chemo-radiotherapy and associated extensive resections, an argument can be made to pursue more aggressive extrathoracic staging even in those without symptoms of distant metastases.
In one of the largest studies published for SSTs at Memorial Sloan-Kettering Cancer Center (61) with patients treated according to the bimodality therapy (preoperative radiotherapy followed by en bloc resection) 5-year survival was 46% for stage IIB, 0% for stage IIIA, and 13% for stage IIIB tumors. Survival was influenced by T and N status and completeness of resection. However, resection was considered pathologically complete in only 64% of T3 N0 and 39% of T4 N0 tumors. Therefore accurate staging significantly influences survival.
Pancoast tumours are staged according to the 2009 IASLC/UICC AJCC TNM staging system for NSCLC (62) (see Table 2).
Summary
Pancoast (or superior sulcus) tumors represent 3% to 5% of all lung cancers, and are biologically similar to typical NSCLC. They are characterized by the infiltration of the thoracic inlet and may present with a constellation of symptoms depending on the invasion of specific structures. The combination of severe and unrelenting shoulder and arm pain along the distribution of the eighth cervical and first and second thoracic nerve trunks, Horner’s syndrome (ptosis, miosis, and anhidrosis) and atrophy of the intrinsic hand muscles comprises a clinical entity named as “Pancoast-Tobias syndrome”. Various other neoplasms or infectious diseases have been reported to be responsible for the reproduction of the Pancoast syndrome. Variations also existed over the past decades among efforts made to describe the superior pulmonary sulcus as a definite anatomic structure. An understanding of the symptoms that associate the invaded anatomical structures and their radiologic documentation is critical in therapeutic planning. Because these tumors by definition involve the chest wall, it is logical that these patients usually present with local rather than systemic manifestations of the disease. In the early stages, shoulder pain represents the most common symptom due to invasion of the parietal pleura, upper ribs, brachial plexus, endothoracic fascia, or the adjacent vertebral bodies. Clinical features depend upon the location and type of structures invaded at the thoracic inlet by the tumor.
Less than 50% of patients with Pancoast tumors are considered resectable lesions at presentation. The remaining is unresectable because of extensive vertebral body involvement, mediastinal nodal disease, or distant metastases. Therefore accurate staging is mandatory since it significantly influences survival.
Histological proof is mandatory upon presence of mediastinal lymph node enlargement on preoperative radiological examinations, since Pancoast lesions associated with mediastinal nodal metastases (positive N2 or N3 disease) have a poor prognosis. Mediastinoscopy and/or anterior mediastinotomy may be decisive in determining extend of the disease and should be strongly considered in these cases.
Invasion of ipsilateral supraclavicular lymph nodes by the tumor is classified as N3 disease. Studies showed that patients with supraclavicular lymph node metastases exhibited better outcome than patients with N2 disease. Evidence suggests that such involvement may have a prognostic significance similar to that of N1 disease. Additional evidence suggests that the biology of Pancoast tumors is indifferent than that of other NSCLC since survival is increased when resection of a Pancoast tumor involves a lobectomy rather than a wedge resection alone.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pancoast HK. Importance of careful roentgen ray investigations of apical chest tumors. JAMA 1924;83:1407.
- Pancoast HK. Superior pulmonary sulcus tumor: tumor characterized by pain, Horner’s syndrome, destruction of bone and atrophy of hand muscles. JAMA 1932;99:1391-6.
- Hare E. Tumor involving certain nerves. London Med Gazette 1838;1:16-8.
- Herbut PA, Watson JS. Tumor of the thoracic inlet producing Pancoast syndrome: a report of seventeen cases and review of the literature. Arch Pathol (Chic) 1946;42:88-103. [PubMed]
- Haas LL, Harvey RA, Langer SS. Radiation management of otherwise hopeless thoracic neoplasm. J Am Med Assoc 1954;154:323-6. [PubMed]
- Chardack WM, MacCallum JD. Pancoast syndrome due to bronchiogenic carcinoma: successful surgical removal and postoperative irradiation: a case report. J Thorac Surg 1953;25:402-12. [PubMed]
- Shaw RR, Paulson DL, Kee JL. Treatment of the superior sulcus tumor by irradiation followed by resection. Ann Surg 1961;154:29-40. [PubMed]
- Hilaris BS, Luomanen RK, Mahan GD, et al. Interstitial irradiation of apical lung cancer. Radiology 1971;99:655-60. [PubMed]
- Detterbeck FC. Pancoast (superior sulcus) tumors. Ann Thorac Surg 1997;63:1810-8. [PubMed]
- Hilaris BS, Martini N, Wong GY, et al. Treatment of superior sulcus tumor (Pancoast tumor). Surg Clin North Am 1987;67:965-77. [PubMed]
- Fuller DB, Chambers JS. Superior sulcus tumors: combined modality. Ann Thorac Surg 1994;57:1133-9. [PubMed]
- Stanford W, Barnes RP, Tucker AR. Influence of staging in superior sulcus (Pancoast) tumors of the lung. Ann Thorac Surg 1980;29:406-9. [PubMed]
- Shahian DM, Neptune WB, Ellis FH Jr. Pancoast tumors: improved survival with preoperative and postoperative radiotherapy. Ann Thorac Surg 1987;43:32-8. [PubMed]
- Johnson DH, Hainsworth JD, Greco FA. Pancoast’s syndrome and small cell lung cancer. Chest 1982;82:602-6. [PubMed]
- Van Houtte P, MacLennan I, Poulter C, et al. External radiation in the management of superior sulcus tumor. Cancer 1984;54:223-7. [PubMed]
- Hatton MQ, Allen MB, Cooke NJ. Pancoast syndrome: an unusual presentation of adenoid cystic carcinoma. Eur Respir J 1993;6:271-2. [PubMed]
- Ohta Y, Toda A, Ohta N, et al. An atypical lung carcinoid tumor resected after induction therapy with involvement of the superior sulcus region: report of a case. Surg Today 2002;32:632-4. [PubMed]
- Chong KM, Hennox SC, Sheppard MN. Primary hemangiopericytoma presenting as a Pancoast tumor. Ann Thorac Surg 1993;55:9. [PubMed]
- Herbut PA, Watson JS. Tumor of the thoracic inlet producing the Pancoast syndrome: a report of seventeen cases and a review of the literature. Arch Pathol (Chic) 1946;42:88-103. [PubMed]
- Rabano A, La Scala M, Hernandez P, et al. Thyroid carcinoma presenting as Pancoast’s syndrome. Thorax 1991;46:270-1. [PubMed]
- Goldman SM, Fajardo AA, Naraval RC, et al. Metastatic transitional cell carcinoma from the bladder: radiographic manifestations. AJR Am J Roentgenol 1979;132:419-25. [PubMed]
- Amin R. Bilateral Pancoast’s syndrome in a patient with carcinoma of the cervix. Gynecol Oncol 1986;24:126-8. [PubMed]
- Chen KT, Padmanabhan A. Pancoast syndrome caused by extramedullary plasmacytoma. J Surg Oncol 1983;24:117-8. [PubMed]
- Mills PR, Han LY, Dick R, et al. Pancoast syndrome caused by a high grade B cell lymphoma. Thorax 1994;49:92-3. [PubMed]
- Dolan G, Smith J, Reilly JT. Extrapulmonary lymphomatoid granulomatosis presenting as Pancoast’s syndrome. Postgrad Med J 1991;67:914-5. [PubMed]
- Vandenplas O, Mercenier C, Trigaux JP, et al. Pancoast’s syndrome due to Pseudomonas aeruginosa infection of the lung apex. Thorax 1991;46:683-4. [PubMed]
- Gallagher KJ, Jeffrey RR, Kerr SM, et al. Pancoast syndrome: an unusual complication of pulmonary infection by Staphylococcus aureus. Ann Thorac Surg 1992;53:903-4. [PubMed]
- Stanley SL Jr, Lusk RH. Thoracic actinomycosis presenting as a brachial plexus syndrome. Thorax 1985;40:74-5. [PubMed]
- Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast’s syndrome. N Engl J Med 1997;337:1370-6. [PubMed]
- Vamos G, Papp A. Pancoast syndrom und lungentuberkulose. Schweiz Z Tuberk 1960;17:423-30. [PubMed]
- Simpson FG, Morgan M, Cooke NJ. Pancoast’s syndrome associated with invasive aspergillosis. Thorax 1986;41:156-7. [PubMed]
- Mitchell DH, Sorrell TC. Pancoast’s syndrome due to pulmonary infection with Cryptococcus neoformans variety gattii. Clin Infect Dis 1992;14:1142-4. [PubMed]
- Winston DJ, Jordan MC, Rhodes J. Allescheria boydii infections in the immunosuppressed host. Am J Med 1977;63:830-5. [PubMed]
- Tobias J. Sindrome ápico-costo-vertebral doloroso por tumor apexiano: su valor diagnostico en el cáncer primitive pulmonar. Rev Med Latino Am 1932;17:1522-56.
- Kubic S. eds. Surgical anatomy of the thorax. Philadelphia: Saunders, 1970.
- Fraser R, Pare J. eds. Diagnosis of diseases of the chest. 2nd Ed. Vol. 4. Philadelphia: Saunders, 1978.
- Paulson DL. Carcinomas in the superior pulmonary sulcus. J Thorac Cardiovasc Surg 1975;70:1095-104. [PubMed]
- Seydel H, Chait A, Gemlich J. eds. Cancer of the lung. New York: Wiley, 1975.
- Netter F. eds. Respiratory system. Vol 7. New York: CIBA, 1979.
- Teixeira JP. Concerning the Pancoast tumor: what is the superior pulmonary sulcus? Ann Thorac Surg 1983;35:577-8. [PubMed]
- Ginsberg RJ, Martini N, Zaman M, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumor. Ann Thorac Surg 1994;57:1440-5. [PubMed]
- Komaki R. Preopareative radiation therapy for superior sulcus lesions. Chest Surg Clin N Am 1991;1:13-35.
- Takasugi JE, Rapaport S, Shaw C. Superior sulcus tumors; the role of imaging. J Thorac Imaging 1989;4:41-8. [PubMed]
- Miller JI, Mansour KA, Hatcher CR Jr. Carcinoma of the superior pulmonary sulcus. Ann Thorac Surg 1979;28:44-7. [PubMed]
- Laissy JP, Soyer P, Sekkal SR, et al. Assessment of vascular involvement with magnetic resonance angiography (MRA) in Pancoast syndrome. Magn Reson Imaging 1995;13:523-30. [PubMed]
- Heelan RT, Demas BE, Caravelli JF, et al. Superior sulcus tumors: CT and MRI imaging. Radiology 1989;170:637-41. [PubMed]
- Maxfield RA, Aranda CP. The role of fiberoptic bronchoscopy and transbronchial biopsy in the diagnosis of Pancoast’s tumor. N Y State J Med 1987;87:326-9. [PubMed]
- Narayan S, Thomas CR Jr. Multimodality therapy for Pancoast tumor. Nat Clin Pract Oncol 2006;3:484-91. [PubMed]
- Paulson DL, Weed TE, Rian RL. Cervical approach for percutaneous needle biopsy of Pancoast tumors. Ann Thorac Surg 1985;39:586-7. [PubMed]
- Yang PC, Lee LN, Luh KT, et al. Ultrasonography of Pancoast tumor. Chest 1988;94:124-8. [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with no small cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [PubMed]
- MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by PET in apparent stage non-small cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:287-93. [PubMed]
- Schmuecking M, Schneider CP, Presselt N, et al. Are timing of chemoradiation and early therapy response as detected by PET prognostic factors of a multimodality treatment approach for NSCLC stage III? (LUCAS-MD) Proc Am Clin Oncol 2007;25:abstr 7532.
- Attar S, Krasna MJ, Sonett JR, et al. Superior sulcus (Pancoast) tumor: experience with 105 patients. Ann Thorac Surg 1998;66:193-8. [PubMed]
- Detterbeck FC, Jones DR, Rosenman JG. Pancoast tumors. In: Detterbeck FC, Rivera MP, Socinski MA, et al. eds. Diagnosis and treatment of lung cancer: an evidence-based guide for the practising clinician. Philadelphia: WB Saunders, 2001:233-43.
- Jones DR, Detterbeck FC. Surgery for stage I non-small cell lung cancer. In: Detterbeck FC, Rivera MP, Socinski MA, et al. eds. Diagnosis and treatment of lung cancer: an evidence-based guide for the practising clinician. Philadelphia: WB Saunders, 2001:177-90.
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [PubMed]
- Vallières E, Karmy-Jones R, Mulligan MS, et al. Pancoast tumors. Curr Probl Surg 2001;38:293-376. [PubMed]
- Paulson DL. Technical considerations in Stage III disease: “the superior sulcus” lesion. In: Delarue NC, Eschapasse H. eds. International trends in general thoracic surgery. Vol I. Philadelphia: Saunders, 1985:121-33.
- Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 2000;119:1147-53. [PubMed]
- Goldstraw P. eds. International Association for the Study of Lung Cancer. Staging handbook in thoracic oncology. Florida: Orange Park, 2009.


