Computed tomography criteria for the use of advanced localization techniques in minimally invasive thoracoscopic lung resection
Introduction
Computed tomography (CT) screening for lung cancer has significantly reduced the mortality from lung cancer in high risk patients by discovering cancer at an early stage of development (1). The increased use of CT screening has led to patients presenting with smaller nodules. These patients are evaluated based on patient and imaging characteristics to determine the probability of the nodule being malignant (2). Patients with a solitary lung nodule that is highly suspicious for primary lung cancer undergo wedge resection, intraoperative pathologic evaluation, followed by a lobectomy, if proven malignant. Some patients with multiple lung nodules need either diagnosis or therapeutic resection of the metastatic cancer to the lung. Traditionally, these patients underwent a thoracotomy followed by palpation of the lung and eventual resection of the lesions. Patients with a nodule that was >5 mm away from the pleura that was <1 cm in size had about 63% chance of needing a thoracotomy to remove the nodule (3). However, the advancement of both video-assisted thoracoscopic surgery (VATS) and robot-assisted minimally invasive procedures has led to the development of advanced localization techniques to avoid thoracotomy.
Advanced localization techniques are performed with either transbronchial or transthoracic localization. The most common transbronchial technique utilizes electromagnetic navigational bronchoscopy to place a fiducial marker with concurrent methylene blue dye injection. On subsequent thoracoscopic examination, the lesion can be identified with methylene blue on direct visualization and the fiducial can be identified with fluoroscopy (4,5). Another option is to perform transthoracic localization with CT-guidance by an interventional radiologist who leaves a hooked wire, dye, microcoil, contrast dye or radiotracer in the nodule immediately prior to surgical resection (6-14). Alternatively, the patient can undergo localization in the operating room using intraoperative cone beam CT followed by resection (15-17). These advanced localization techniques take additional time and carry additional risks to patients compared to visually identifying the lesion.
Upon review of recent studies that advocate for the use of these techniques, some lung nodules included in the reviewed studies were either abutting the pleural surface or greater than 2 cm in size, factors (7,8,10), criteria that may have eliminated the need for advanced techniques. Moreover, there are no clear CT criteria to help decide when advanced techniques should be employed. In our study, we aim to show that most lung nodules can be removed using visual identification and that small non-solid nodules that are far from the pleura should prompt the use of advanced techniques.
Methods
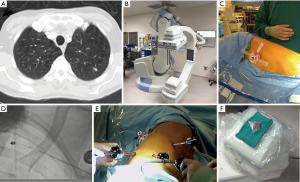
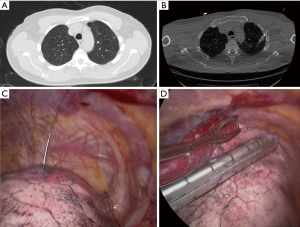
The Institutional Review Board at Houston Methodist Research Institute approved this study (000136980). We performed a retrospective case control study evaluation for patients who underwent resection of a nodule. Inclusion criteria for patients in this study were patients who had either solitary or multiple lung nodules who underwent wedge resection from 2016–2017. A surgeon made a decision based on the nodule’s appearance on the CT scan whether to use advanced techniques to localize the lesion. We collected demographics, medical history, surgical details, and outcomes of the operation from the prospective database for the Society of Thoracic Surgeons at Houston Methodist Hospital, which is extracted from the patients’ electronic health records. We also performed retrospective analysis of the preoperative CT scans to determine the size of the nodule, density of the nodule (solid or ground glass) and the distance to the nearest pleural surface. We compared the characteristics of patients who required advanced localization to patients who had direct identification of the nodule. Three different advanced localization techniques were used during this study. We performed cone beam CT localization in a hybrid operating room, where we performed both localization and resection after the patient underwent general anesthesia. First, the patient underwent localization of the nodule with a needle and/or fiducial under cone beam CT (Figure 1). The patient then underwent minimally invasive wedge resection of the nodule and based on the pathology, the patient underwent additional procedures (i.e., lobectomy). Another intraoperative localization technique was transbronchial localization, in which we used electromagnetic navigational bronchoscopy to perform intraoperative localization of the nodule. With a single lumen endotracheal tube, we would place fiducial and methylene blue with fluoroscopy. Then, a double lumen endotracheal tube was placed, the patient was placed in lateral decubitus position, and we performed minimally invasive wedge resection of the lesion. Based on the pathologic assessment, the patient underwent additional procedures. Finally, we also utilized preoperative CT localization with a hook wire in the Radiology department. The patient had this procedure immediately prior to operation and subsequently the patient had a minimally invasive procedure to remove the lesion (Figure 2).
Demographic and clinical data were reported as frequencies and proportions for categorical variables. Continuous variables with normally contributed data were presented as mean ± standard deviation (SD). Continuous variables with non-normally contributed data were presented as median and interquartile range (IQR). Univariable and multiple logistic regression analyses were performed to determine the characteristics associated with whether the patient required advanced techniques to perform resection of the lung nodule. Variables that had a P value of <0.2 in the univariable analysis or considered clinically significant were investigated further by multiple logistic regression modeling. Variable selection for the multiple logistic regression models was conducted using the Bayesian model averaging (BMA) method (18,19). The best model was selected based on the smallest Bayesian information criterion (BIC). We determined the model discrimination by the area under the receiver operating characteristic (ROC) curve (AUC). We assessed the model calibration using the Hosmer-Lemeshow goodness-of-fit test with a non-significant P value indicating a good calibration. The diagnostic performance of individual predictors of the final model as well as different combinations of these predictors were also evaluated using the sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, and AUC (95% CI). All analyses were performed on Stata version 14.2 (StataCorp LCC, College Station, TX, USA). A P value of <0.05 was considered statistically significant.
Results
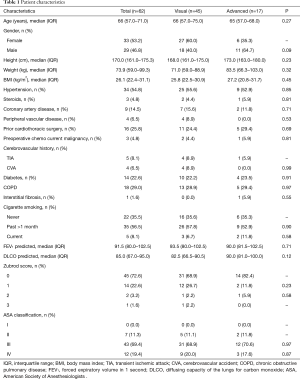
There were 62 patients who met the inclusion criteria. Of these, 45 patients (73%) had their lung nodule resected by direct identification and 17 patients had advanced localization techniques to identify the nodule. Eleven patients had cone beam CT localization and resection of the nodule. Three patients had lung resection after localization with electromagnetic navigation and 3 patients had preoperative CT guided localization of the lung nodule. The group’s median age was 66 years old with 33 women (53%) and 29 (47%) men. There were no significant differences in co-morbidities, Zubrod score, American Society of Anesthesiologists (ASA) classification, smoking status and pulmonary function test (Table 1).
Full table
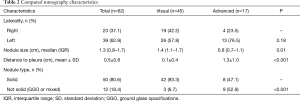
However, there were significant differences in the lung nodule’s characteristics on the CT scan. Patients who had resection of the lung nodule based on direct identification had significantly larger lung nodules with the median size of the nodule at 1.4 cm compared to 0.8 cm for patients who had the nodule identified with advanced techniques (P=0.01). Moreover, nodules resected by direct inspection had a median distance to the pleura of 0.1 cm, compared to nodules resected with advanced localization, which were a median of 1.3 cm from the pleura (P<0.001). Finally, nodules which required advanced localization techniques were more likely to be ground glass rather than solid than those resected by direct inspection (Table 2).
Full table
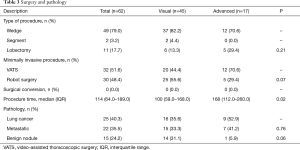
All patients underwent minimally invasive surgery to remove the lung nodule with either VATS (52%) or robot-assisted techniques (48%). Typically patients had two 5-mm Xcel ports and one 12-mm Xcel port for VATS wedge resection. Patients who underwent subsequent VATS lobectomy had three port incision technique in which the patient had a 4-cm utility incision in the 4th or 5th intercostal space in the mid-axillary line, 12-mm posterior port at the 7th intercostal space, posterior to the posterior axially line and 12-mm anterior port in the 7th intercostal space mid-axillary line. We used “five on a dice” port placement for the robot-assisted technique (20,21). There were no surgical conversions to a thoracotomy. Most patients needed only a wedge resection (49, 79.0%), while 2 patients (3.2%) had segmentectomy after the wedge and 11 patients (18%) had a lobectomy following the wedge resection. At final pathology, only 15 (24%) patients had benign nodules, 22 (36%) had metastatic disease and 25 (40%) had primary lung cancer. There was no difference between the visualized group and the advanced technique group for surgical and pathologic characteristics (Table 3). Only 8 patients (13%) had grade 2 or higher complications after surgery. The most common complications were urinary retention (8, 13%), discharge with a Foley catheter (3, 5%) and urinary tract infection (3, 5%). All patients went home after surgery with a mean length of stay of 2.8 days and a readmission rate of 8.1% (n=5) within 30 days (Table 4).
Full table

Full table
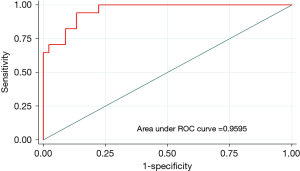
Multiple logistic regression showed that nodule size, distance to the pleura and a nodule that was not solid were predictive factors for the use of advanced techniques to localize the lung nodule prior to resection. Factors including having all small lung nodules, a nodule located far away from the pleura, or non-solid nodules on the CT scan had a sensitivity of 77% and specificity of 91% in predicting the use of advanced techniques to localize the nodule. Our analysis of the area under the curve showed that a solitary lung nodule that is greater than 1.3 cm and less than 5 mm from the pleura can be removed with direct localization technique with 96% probability of success (Figure 3).
Discussion
CT screening for patients at high risk for developing lung cancer has led to an increase in the number of patients with small lung nodules suspicious for malignancy. Often, these nodules are not anatomically amenable to either transbronchial or transthoracic biopsy. In the past, these patients would undergo open thoracotomy and wedge resection and, based on the nodule pathology, a subsequent appropriate resection such as a lobectomy. However, with significant improvements in outcomes using minimally invasive surgery, new techniques have been developed to help identify these nodules.
Transbronchial localization of the nodule has the advantage of being performed efficiently at the time of surgery. The major disadvantage of the localization process is that it is not as accurate as CT localization. CT guided wire localization of the lesion can be performed prior to surgery. After localization, the patient is then taken to the operating room where they undergo minimally invasive wedge resection of the lung around the localization wire. The advantage of this technique is more accurate localization and no additional time spent localizing the nodule in the operating room. However, the patient must undergo a separate localization in a different department of the hospital and the procedure has its own set of potential complications. Patients have been known to have pneumothorax and hemorrhage from this procedure (12,14). There is also the possibility of dislodging the wire prior to surgery, which would result in inaccurate localization of the lesion. Cone beam CT localization allows for accurate intraoperative localization and provides an opportunity to re-localize the nodule if it is not seen in the initial specimen. However, the major disadvantage of the procedure is the additional time needed in the operating room to localize the lesion.
Although these advanced techniques are available, not all nodules require these techniques. Our study demonstrates that the large majority (73% in this study) of nodules can be resected with direct visual and tactile localization alone. However, the nodules where advanced techniques were employed were significantly smaller, further away from the pleura and less likely to be solid. Having all three characteristics significantly increased the likelihood that advanced techniques would be used to perform the resection. None of the patients required conversion to open thoracotomy, showing that judicious use of advanced techniques can help achieve minimally invasive pulmonary resection in this group of patients. The CT criterion of a solid pulmonary lesion greater than 1.3 cm that is less than 5 mm from the pleura can be used as a concrete cutoff to decide when to use direct visual and tactile localization instead of advanced techniques. The major limitation of this study is that it is a retrospective design examining a single institutional experience by a single surgeon looking at small lung nodules undergoing initial wedge resection. A prospective study examining this defined CT criteria prescribing the use of advanced techniques will provide more clarity with regard to the success rate of this recommendation.
Conclusions
In summary, when planning minimally invasive resection of a pulmonary nodule, advanced localization techniques should be considered for nodules which are small in size (<1.3 cm), further away from the pleura (>5 mm) and which are not solid in CT appearance.
Acknowledgements
We thank Ann Saikin for language editing of the manuscript.
Footnote
Conflicts of Interest: MP Kim has consulted for Medtronics, Boston Scientific, Olympus and Intuitive Surgical; EY Chan has consulted for Medtronics, Boston Scientific and Olympus. The other authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board at Houston Methodist Research Institute approved this study (000136980) and written informed consent was waived.
References
- National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Chan EY, Gaur P, Ge Y, et al. Management of the Solitary Pulmonary Nodule. Arch Pathol Lab Med 2017;141:927-31. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Bolton WD, Richey J, Ben-Or S, et al. Electromagnetic Navigational Bronchoscopy: A Safe and Effective Method for Fiducial Marker Placement in Lung Cancer Patients. Am Surg 2015;81:659-62. [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Hsu HH, Shen CH, Tsai WC, et al. Localization of nonpalpable pulmonary nodules using CT-guided needle puncture. World J Surg Oncol 2015;13:248. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Pang X, Xue L, Chen J, et al. A novel hybrid technique for localization of subcentimeter lung nodules. J Thorac Dis 2017;9:1107-12. [Crossref] [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007;106:911-8. [Crossref] [PubMed]
- Hsieh CP, Hsieh MJ, Fang HY, et al. Imaging-guided thoracoscopic resection of a ground-glass opacity lesion in a hybrid operating room equipped with a robotic C-arm CT system. J Thorac Dis 2017;9:E416-9. [Crossref] [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923-36. [Crossref] [PubMed]
- Wasserman L. Bayesian Model Selection and Model Averaging. J Math Psychol 2000;44:92-107. [Crossref] [PubMed]
- Kim MP, Chan EY. “Five on a dice” port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J Thorac Dis 2017;9:5355-62. [Crossref] [PubMed]
- Khan N, Fikfak V, Chan EY, et al. “Five on a Dice” Port Placement Allows for Successful Robot-Assisted Left Pneumonectomy. Thorac Cardiovasc Surg Rep 2017;6:e42-4. [Crossref] [PubMed]


