Regulatory T cells may play a protection role in postoperative pulmonary dysfunction in rheumatic heart disease
Introduction
An inflammatory response and ischemic-reperfusion that are mediated by extracorporeal circulation are commonly observed in patients after cardiac surgery and are closely related to each other. The causes of the inflammatory response were initially addressed by Butler et al. in 1993 (1). Later, it was widely recognized that the process of cardiopulmonary bypass (CPB) can activate patients’ major host defense pathways, which can induce coagulation, fibrinolysis, activation of complement, and the release of leukocytes, adhesion molecules, and multiple inflammatory mediators. Eventually, the excessive release of these inflammatory mediators can trigger signaling cascades that may cause organ dysfunction.
The lung is the most vulnerable organ to the inflammatory response and the ischemic-reperfusion injury following CPB (2,3). PPD is the most common complication after cardiac surgery with CPB, which can have variable outcomes, ranging from mild hyoxemia, abnormal gas exchange or even acute respiratory distress syndrome (ARDS). PPD may prolong the length of the ICU stay and lead to increasing morbidity and mortality. According to a database that included 5,798 patients that had undergone cardiac surgery, 9.1% of patients required mechanical ventilation for at least 72 h after surgery. The mortality rate of these cases was 5.5 times higher than those that did not require prolonged ventilation (4).
Many interventions have been proposed to attenuate the inflammatory response after CPB, such as the use of minimized extracorporeal circulation, biocompatible circuit coating, pharmacological intervention, improved surgical and anesthesia techniques and others. These methods have been proved to be effective to some degree. However, recent developments in immunology have indicated that potential injury could be prevented by controlling the balance of pro- and anti-inflammatory responses.
Treg cells are a subpopulation of T cells. A previous study revealed that CD4+CD25+CD127low Treg cells can accurately represent the functional Treg cells and can be easily detected by flow cytometry (5). Treg cells are highly immunosuppressive and play an important role in self-tolerance by patients with auto-immune disorders, such as allergy, cancer, transplantation and inflammatory diseases.
By contrast, Th17 cells mainly have a pro-inflammatory role, since they mediate the release of the IL-17A, IL-17F, IL-21 and IL-22 cytokines (6). These cytokines are known to up-regulate immune reactions, with IL-17 having the strongest effect. IL-17 receptors are expressed on the surface of epithelial cells (6), and their activation can recruit neutrophils to the site of inflammation to clear infections (7). However, persistent immune responses may lead to aggravation of the inflammatory reaction and cause further tissue damage (8).
Treg and Th17 cells have opposite functions in inflammatory responses, although their origin and differentiation are closely related. Various research publications have reported that the Treg and Th17 cells play a pivotal role in many diseases (9-11). As a significant component of the immune system, we hypothesize that the Treg and Th17 cells are also involved in the occurrence of PPD in RHD patients, and we speculate that the immune condition of patients may predict the severity of PPD.
Methods
Subjects and protocol
This prospective observational study was conducted at the cardiac surgery department at the Shanghai Chest Hospital. Consecutive patients (18≤ age ≤75 years) who met the diagnosis of RHD and were undergoing elective valve replacement or valvoplasty surgery with CPB were recruited for this study. Patients with lung dysfunction before surgery, low left ventricular ejection fraction (EF <35%) before or after surgery, and patients with diabetes, tumor, exogenous hormone therapy, organ dysfunction, emergency operation, and acute infection in combination with coronary bypass grafting were excluded from this study. The primary endpoint of this study was mortality or severe organ dysfunction.
Fifty-six adult patients were recruited for this study between October 2015 and April 2016. Thirty-one patients underwent single valve replacement or valvoplasty surgery, aortic valve replacement (n=12), mitral valve replacement (n=8), tricuspid valve surgery (n=2), mitral valvoplasty (n=8), and tricuspid valvoplasty (n=1); 19 patients underwent double valve repair and/or valvoplasty surgery, mitral and tricuspid valvoplasty (n=5), mitral valve replacement and tricuspid valvoplasty (n=10), aortic valve replacement and tricuspid valvoplasty (n=4); 6 patients underwent aortic and mitral valve replacement and tricuspid valvoplasty surgery.
The severity of PPD at 24 h after surgery was classified in accordance with the Berlin definition (12) for ARDS:
- Oxygenation index
- No: PaO2/FiO2 ≥300 mmHg (n=21);
- Mild: 200 mmHg < PaO2/FiO2 ≤300 mmHg (n=27);
- Moderate: 100 mmHg < PaO2/FiO2 ≤200 mmHg (n=8);
- Severe: PaO2/FiO2 ≤100 mmHg (n=0).
The worst oxygenation during the first 24 h after surgery was used to determine the occurrence and severity of ARDS.
- An additional radiographic evaluation was performed when the OI of patients was lower than 300 mmHg. The presence of bilateral opacities was judged to confirm the diagnosis of ARDS.
- The data were collected during the first 24 h after surgery.
- Cardiogenic PPD was excluded from this study.
Data collection and risk score calculation
Each patient’s demographic information such as age, sex, body mass index (BMI), EF, EuroSCORE II (13), and NYHA class was recorded. In addition, the perioperative laboratory and clinical data, such as CPB time, aortic clamp time, auxiliary circulation time, transfusion volume, lowest Hct, ventilation time, cardiac surgery ICU LOS, and the oxygenation index (PaO2/FiO2) at 2 h (PaO2/FiO2-1) and 24 h (PaO2/FiO2-2) after admission into the ICU were collected.
Management of CPB and anesthesia
All patients received the same type of anesthesia. The routine extracorporeal circulation included two venous cannulas, an aortic cannula, a left atrial vent tube, an oxygenator, and a roller pump. Patients received 300 IU/kg of heparin before cannulation of the aorta, and, during the surgery, additional heparin was administered, accordingly, to maintain an activated coagulation time of at least 480 seconds. The CPB was performed under mild hypothermia (35 °C) with the flow rate adjusted to 2.2–2.8 L/(min˙m2). The cold blood cardioplegia (4 °C) was infused routinely through the aortic root cannula after aortic clamping (except for severe aortic valve regurgitation or aortic root surgery). To prevent thrombosis, heparin (300 U/kg) was used for all patients before cannulation, targeting a 400-s activated clotting time. Additionally, the anticoagulation effect was neutralized by protamine after the removal of cannulas.
Blood sampling and measurements
Six milliliters of peripheral blood was withdrawn from each of the 56 patients before anesthetization, 30 minutes after heparin neutralization, and 24, 72, and 120 h after surgery. Plasma was separated from blood cells through centrifugation and stored at −80 °C for measurement of cytokine content. The blood cells were directly used for the isolation of peripheral blood mononuclear cells.
Flow-cytometric analysis
The quantity of Treg and Th17 cells was calculated by flow-cytometry. A percentage of Treg and Th17 cells from peripheral blood mononuclear cells was freshly isolated and stored at 37 °C for flow cytometry. Treg cells were stained with FITC-CD4, APC-CD25, and PE-CD127 antibodies (BD, Pharmingen, San Diego, CA). The Th17 cells were stained with FITC-CD3 and APC-CD8 antibodies at first. Cells were then permeabilized and fixed with a fix/perm kit (BD, Pharmingen, San Diego, CA) and stained with PE-IL17A antibody (BD, Pharmingen, San Diego, CA). The differentially labelled cells were sorted and counted with a BD FACSCalibur, and the results were analyzed with the cell analysis FlowJo software. The percentages of Treg and Th17 cells in blood samples were recorded separately as Treg/Th17-1/2/3/4/5 according to the different time points.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-10 and IL-17A were measured by the hypersensitive ELISA kit (ELISA, eBioscience) according to the manufacturer’s instructions. Additionally, the concentrations of IL-10 and IL-17 in the blood samples were recorded separately as IL-10/IL-17-1/2/3/4/5 according to the different time points.
Treg inhibitory function assay
The CD4+CD25+ (Treg) cells and the CD4+CD25− effective T cells (Teff) cells were collected from the peripheral blood mononuclear cells by the EasySep™ Human CD4+CD25+ T Cell Isolation Kit (Stemcell, Technologies, Vancouver, BC, Canada). The Teff cells were incubated with carboxyfluorescein diacetate succinimidyl ester (CFSE) and 5% CO2 for 15 min at 37 °C. For the Treg suppression analysis, the Teff cells were single cultured and then co-cultured with Treg cells at a ratio of 1:1. The isolated T cells were activated by the CD3/CD28 dynabeads (Thermo, Scientific) and cultured with IL-2 (400 IU/mL) in a total PRMI-1640 medium. The Teff proliferation rate was measured by the FACSCalibur, and these values were used to represent the Tregs inhibitory function.
Statistical analysis
All of the continuous variables are presented as the mean ± SD or median, and the categorical variables are expressed as absolute values. The data were analyzed by GraphPad Prism 5.0 (GraphPad software, San Diego, CA, USA) or SPSS 22.0 (SPSS software, Chicago, USA). For the comparison of data in the no/mild/moderate ARDS groups, a one-way-ANOVA was applied to normally distributed data, and the Kruskal-Wallis test was applied to non-normally distributed data. Correlations were investigated by the Pearson’s (normal distribution variables) or the Spearman’s (non-normal distribution variables) test analysis. The receiver operating curve (ROC) was used to evaluate the diagnostic value of the parameters recorded in this study. All of the tests were two-tailed, and a P value <0.05 was considered statistically significant.
Results
Baseline characteristics and clinical data of patients
Between October 2015 and May 2016, a total of 56 patients were recruited for this study. Table 1 shows the basic characteristics and clinical data of patients grouped according to the severity of their lung injury at 24 h after surgery. As shown in Table 1, no significant differences were found in gender, BMI, EuroSCORE II, NYHA class and reoperation rate among each group of patients before surgery (P=0.982, 0.145, 0.360, 0.140, 0.369). Additionally, the CPB time (P=0.115), auxiliary circulation time (P=0.213), transfusion rate (P=0.151) and lowest Hct (P=0.965) during surgery were not statistically different. By contrast, patients of older age (P=0.014) and with lower EF (P=0.002) before surgery and longer aortic-clamp time (P=0.001) during surgery were more likely to develop severe PPD at 24 h after surgery.
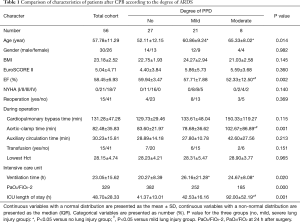
Full table
Correlation of the circulating Treg and Th17 cells and their associated cytokine levels with PPD severity
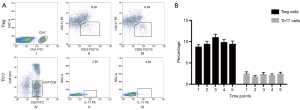
The number of Treg and Th17 cells was analyzed by flow cytometry. The percentage of CD4+CD25+CD127low Treg cells increased while the IL17A+Th17 cells decreased after experiencing CPB in almost all patients recruited in the study (Figure 1).
As shown in Table 2, the proportion of Tregs in the total CD4+ T cells before anesthesia induction and after heparin neutralization was decreased in patients with severe PPD. By contrast, the ratio of Treg/Th17 before anesthesia was high in patients with milder PPD. The concentration of IL-10 had no statistical significance among the three groups at different time points. By contrast, the concentration of IL17A was significantly higher in the moderate lung injury group.
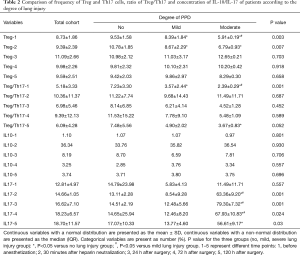
Full table
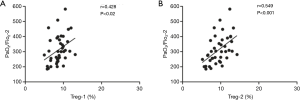
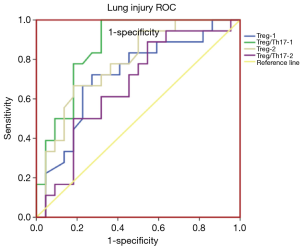
A Spearman’s correlation analysis of the frequency of Treg cells with the corresponding PaO2/FiO2 is shown in Figure 2. The frequencies of Treg-1/2 were positively correlated with PaO2/FiO2-2 (r=0.428, P<0.02; r=0.549, P<0.001). As shown in Figure 3, the AUC calculation for Treg-2 was 0.787 (95% CI: 0.639 to 0.934, P<0.002), which is the best measurement for predicting severe PPD at 24 h after admission into the ICU.
The perioperative function of Treg cells
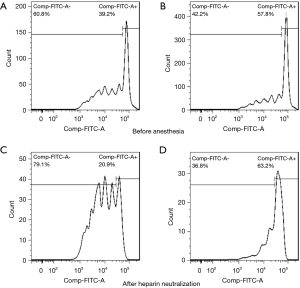
The inhibitory function of Treg cells was determined by measuring the proliferative ability of Teff cells after co-incubation with Treg cells. Our results revealed that the inhibitory function of Treg cells on the proliferation of Teff cells was improved 30 min after heparin neutralization compared to measurements prior to anesthesia induction (Figure 4). Specifically, before anesthesia, a 20.45%±3.78% reduction in the Teff proliferation rate was observed after co-culturing with Treg cells, and this rate was further reduced (36.27%±4.34%) after heparin neutralization.
Discussion
Lung function is the main factor that affects patient’s recovery time after surgery and mortality (14). Although the pathogenesis of inflammation and lung injury is variable, there is sufficient evidence to indicate that inflammatory cells and signaling mediators play a key role in this process (15). In this study, we have focused our interest on investigating the role of the Treg and Th17 cells in lung injury after CPB.
Regulatory T cells maintain self-tolerance and immune homeostasis through their immunosuppressive capabilities. Treg cells’ contact-independent suppressive mechanisms are mainly mediated by the production of inhibitory cytokines (e.g., IL-10, TGF-β and IL-35) (16). In addition, these cells can protect tissue from injury through tissue repair mechanisms or by limiting inflammatory responses (17). Treg cells were first discovered by researchers working with the bronchoalveolar lavage fluid of acute lung injury in mice and human patients. A subsequent study demonstrated that Treg cells respond to lung injury by releasing TGF-β and accelerating neutrophil apoptosis (18). However, excessive activation of neutrophils via production of pro-inflammatory cytokines and reactive oxygen species can cause cellular and endothelial damage, which is the main pathogenic factor of lung injury (19,20). Researchers investigating sepsis-induced lung injury reported that the percentage of Treg cells was relatively increased in survivors when compared to dead patients (21,22). Similarly, in our study, we also observed that the increased percentage of Treg cells and the higher ratio of Treg/Th17, before anesthesia and at 30 min after heparin neutralization, correlated with milder PPD after extracorporeal circulation. This protective role of Treg cells could be attributed to the release of IL-10, since it has been shown that this cytokine prevents the overwhelming specific or unspecific immune reactions and tissue damage by inhibiting the production of inflammatory mediators and the presentation of antigens (23). However, our research did not identify any obvious association between the IL-10 levels and the post-CPB PPD, and this is in accordance with an earlier study (22). Nevertheless, the mechanism by which Treg cells are implicated in the development of CPB-mediated PPD requires further investigation.
The Th17 cells are characterized by their ability to release IL-17 cytokines. Inhibition of IL-17 by anti-rat IL-17 antibody after lung multi-trauma alleviated acute lung inflammation (24), supporting a pro-inflammatory role for IL-17, as well as suggesting that it can be a potential therapeutic target for the treatment of lung PPD. In patients with ARDS, the elevated circulation and alveolar levels of IL-17A were correlated with increased alveolar permeability and neutrophil levels (25), suggesting that IL-17A may cause tissue damage through recruitment of neutrophils. In our study, we also found an apparent increase of IL-17A levels in the severe PPD group after extracorporeal circulation. However, there was no correlation between Th17 cells and IL-17A levels, which suggests that IL-17A is also secreted by other types of cells, such as CD8+ T cells, natural killer T (NKT) cells, monocytes, and dendritic cells (DC) (26). Therefore, more research is required to reveal the mechanism of IL-17A, which could potentially lead to the development of a novel therapeutic strategy for the treatment of PPD after CPB.
Prior studies indicated that CPB can promote the differentiation of T-helper (TH) cells. Following CPB, the Th1 cells that mediate pro-inflammatory immune responses were temporally down-regulated, while the Th2 cells, which mediate anti-inflammatory immune responses, were up-regulated (27,28). In our study, we have shown that the percentage of Treg cells in the CD4+ T cells was slightly increased, unlike that of the Th17 cells, which was decreased. In addition, we have revealed that the inhibitory function of Treg cells on the effector T cells was also improved after CPB. Our results supplement the findings of earlier research studies and further prove that the body is in an immunosuppressive state after CPB surgery. The up-regulated anti-inflammatory response may be the mechanism of the body’s immune system to protect the host from the damage caused by the overwhelming inflammatory response after cardiac surgery with CPB. Furthermore, the body’s immunosuppressive state does not necessarily increase the possibility of infection as has been previously hypothesized.
A surprising finding is that the increased percentage of Treg cells in the CD4+ T cell population and the higher ratio of Treg/Th17 before the induction of anesthesia may be correlated with increased respiratory function after cardiac surgery. The difference of the immune function of patients may correlate with RHD—an autoimmune progressive destructive valvular disorder. Researchers have shown that the function and quantity of Treg cells in RHD patients are not only related to a person’s health but are also different among patients (29,30). Possibly, the stronger the immune regulation capability possessed by the patient, the less damage the lung will suffer after cardiac surgery. This could explain why patients with no difference in preoperative risk assessment and that had experienced almost the same procedure suffered from different degrees of PPD. Additionally, the Berlin definition of ARDS can estimate the severity of the respiratory condition of patients sufficiently well (12) and can be used to guide the clinical treatment and predict the mortality rate to some degree. By contrast, our study found that the higher percentage of Treg cells in CD4+ T cells, which play an immunosuppressive role in the immune system before cardiac surgery, may well predict the degree of PDD. However, due to limitations of patient numbers, this should be investigated further; therefore, more research is required in the future.
Conclusions
In summary, Treg cells play a protective role in PPD in RHD patients undergoing cardiac surgery with CPB. The higher proportion of Treg cells in the total CD4+ T cell population and higher Treg/Th17 ratio before anesthesia induction and 30 min after heparin neutralization can partially protect patients from a severe inflammatory response and lung injury. Treg cell quantification can be beneficial for predicting and treating lung injury after CPB-mediated cardiac surgery.
Acknowledgements
We are deeply grateful for the cooperation of the individuals who participated in the study and the priceless assistance from the nurses in the cardiac surgery department of the Shanghai Chest Hospital.
Funding: This work was supported by the Science and Technology Commission of Shanghai Municipality, Medical Guidance Funded Project (grant number: 09411966000), the National Nature Science Foundation of China (grant numbers: 31370904, 81671579), the program for scientific and technological innovation from the Science and Technology Commission of Shanghai Municipality (15401900500) and Shuguang Planning of Shanghai Municipal Education Commission of (16SG14).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
Ethical Statement: Ethical approval for this clinical trial was obtained from the Clinical Trial Institute of the Shanghai Chest Hospital. All the procedures performed in the study that involved human participants were in accordance with the ethical standards of the institutional and national research committee and in compliance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1993;55:552-9. [Crossref] [PubMed]
- Miller BE, Levy JH. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997;11:355-66. [Crossref] [PubMed]
- Richter JA, Meisner H, Tassani P, et al. Drew-Anderson technique attenuates systemic inflammatory response syndrome and improves respiratory function after coronary artery bypass grafting. Ann Thorac Surg 2000;69:77-83. [Crossref] [PubMed]
- Filsoufi F, Rahmanian PB, Castillo JG, et al. Predictors and early and late outcomes of respiratory failure in contemporary cardiac surgery. Chest 2008;133:713-21. [Crossref] [PubMed]
- Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151-64. [PubMed]
- Aggarwal S, Xie MH, Maruoka M, et al. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res 2001;21:1047-53. [Crossref] [PubMed]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454-67. [Crossref] [PubMed]
- Bordon J, Aliberti S, Fernandez-Botran R, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis 2013;17:e76-83. [Crossref] [PubMed]
- Jiang H, Wu X, Zhu H, Xie Y, Tang S, Jiang Y. FOXP3(+)Treg/Th17 cell imbalance in lung tissues of mice with asthma. Int J Clin Exp Med 2015;8:4158-63. [PubMed]
- Marshall EA, Ng KW, Kung SH, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer 2016;15:67. [Crossref] [PubMed]
- Wang D, Huang S, Yuan X, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol 2017;14:423-31. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Altmay E, Karaca P, Yurtseven N, et al. Continuous positive airway pressure does not improve lung function after cardiac surgery. Can J Anaesth 2006;53:919-25. [Crossref] [PubMed]
- Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg 1997;84:920-35. [Crossref] [PubMed]
- Pandiyan P, Zheng L, Ishihara S, et al. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007;8:1353-62. [Crossref] [PubMed]
- Arpaia N, Green JA, Moltedo B, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015;162:1078-89. [Crossref] [PubMed]
- D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009;119:2898-913. [Crossref] [PubMed]
- Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003;31:S195-9. [Crossref] [PubMed]
- Miyashita T, Ahmed AK, Nakanuma S, et al. A Three-phase Approach for the Early Identification of Acute Lung Injury Induced by Severe Sepsis. In vivo 2016;30:341-9. [PubMed]
- Song H, Zhou Y, Li G, et al. Regulatory T cells contribute to the recovery of acute lung injury by upregulating Tim-3. Inflammation 2015;38:1267-72. [Crossref] [PubMed]
- Yu ZX, Ji MS, Yan J, et al. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care 2015;19:82. [Crossref] [PubMed]
- Sabat R, Grutz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev 2010;21:331-44. [Crossref] [PubMed]
- Iwakura Y, Ishigame H, Saijo S, et al. Functional specialization of interleukin-17 family members. Immunity 2011;34:149-62. [Crossref] [PubMed]
- Mikacenic C, Hansen EE, Radella F, et al. Interleukin-17A Is Associated With Alveolar Inflammation and Poor Outcomes in Acute Respiratory Distress Syndrome. Crit Care Med. 2016;44:496-502. [Crossref] [PubMed]
- Shabgah AG, Fattahi E, Shahneh FZ. Interleukin-17 in human inflammatory diseases. Postepy Dermatol Alergol 2014;31:256-61. [Crossref] [PubMed]
- Markewitz A, Lante W, Franke A, et al. Alterations of cell-mediated immunity following cardiac operations: clinical implications and open questions. Shock 2001;16 Suppl 1:10-5. [Crossref] [PubMed]
- MacCallum NS, Finney SJ, Gordon SE, et al. Modified criteria for the systemic inflammatory response syndrome improves their utility following cardiac surgery. Chest 2014;145:1197-203. [Crossref] [PubMed]
- Mukhopadhyay S, Varma S, Gade S, et al. Regulatory T-cell deficiency in rheumatic heart disease: a preliminary observational study. J Heart Valve Dis 2013;22:118-25. [PubMed]
- Abdul-Auhaimena N, Al-Kaabi ZI. Functional and Developmental Analysis of CD4(+)CD25(+) Regulatory T Cells under the Influence of Streptococcal M Protein in Rheumatic Heart Disease. Iran J Med Sci 2011;36:122-7. [PubMed]


