Nivolumab induced vitiligo-like lesions in a patient with metastatic squamous cell carcinoma of the lung
Introduction
Nivolumab, a fully humanized monoclonal IgG4 antibody blocking programmed cell death-1 (PD-1), has demonstrated improved survival over docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) (1,2). Vitiligo-like depigmentation is a well described immune-related adverse event (irAE) in melanoma patients receiving immunotherapy with PD-1 inhibitors but it’s rare in NSCLC (3). We experienced a rare case of metastatic NSCLC who developed vitiligo-like lesions during nivolumab treatment after excess sunburn. We presented clinical course of this patient with the data of histological and immunohistochemical analysis of the skin lesions.
Case presentation
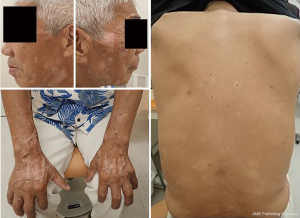
A 69-year-old former-smoking male was diagnosed with metastatic squamous cell carcinoma of the lung in 2015. The patient had a medical history of diffuse large B cell lymphoma of the right testicle which had been surgically resected in 2014 and being observed without recurrence. His hobby was gardening, and he enjoyed it without wearing sunscreen. He underwent chemotherapy with cisplatin and gemcitabine as the first line therapy for NSCLC, however the regimen was discontinued due to unacceptable toxicity. Because of disease progression after 2 additional chemotherapy regimens, he started 4th line therapy with nivolumab of 3 mg/m2 every 2 weeks on February 3, 2016. A dramatic response was observed in 2 months. In July 2017 of 15th month of nivolumab, the patient developed vitiligo-like lesions localized on insolated areas (face, neck, forearms, and hands) (Figure 1). Detailed examination of the whole body revealed skin depigmentation also occurred in his trunk. The patient had no personal and family history of vitiligo, thyroiditis, underlying skin or autoimmune disorders, recent exposure to radiation, and no new medications. A few senile skin pigmentations were observed on his face and limbs, but it did not have a change during anti-PD-1 treatment. There was not an abnormal nevus on his body. Furthermore, a dermatologist examined and diagnosed him.
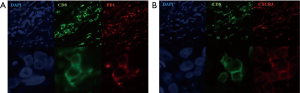
A punch biopsy was undertaken from 3 sites: vitiligo-like lesions, perilesional skin, and normal skin. Histopathological examination of vitiligo-like lesions and perilesional skin revealed loss of melanocytes and melanin pigment. Meanwhile, the massive invasion of epidermal tissues by CD8+ T cells was observed in the macroscopically normal skin adjacent to vitiligo-like lesions (Figure 2). These inflammatory cells were sparse in vitiligo-like lesions themselves. Immunofluorescence analysis characterized these infiltrating CD8+ T cells to express CXCR3 (Figure 3). The vitiligo-like lesions rapidly spread throughout the body. Topical corticosteroids were not effective for the lesions. No systemic treatments were administered for the adverse effect. He is still receiving treatment with nivolumab with no another adverse event, and experiencing continued good response during subsequent 9 months until now.

Discussion
Vitiligo is characterized by acquired white patchy depigmentation by the loss of melanocytes, and has long been suspected to be an autoimmune disease (4). The onset of vitiligo can be triggered by many factors such as sunburn, mechanical trauma and chemical exposures to the melanocytes; the triggers are all thought to induce oxidative stress, which increase melanocyte targeting by promoting antigen presentation, resulting progressive development of white macules on the skin. However, the trigger is unclear in most cases (4,5). Vitiligo-like lesions are sometimes observed in melanoma patients receiving anti-PD-1 therapies, and considered to be ascribed to cross-reaction of anti-melanoma immunity to normal melanocyte antigens. In melanoma patients, vitiligo-like depigmentation has onset between 2 and 15 (median, 4–5) months from the start of anti-PD-1 therapy, and is generally associated with favorable clinical outcomes (6,7). Vitiligo is not life-threatening, but has been shown to exert a harmful influence on the quality of life. A recent meta-analysis showed the patients with vitiligo were significantly more prone to suffer from depression (8).
Larsabal et al. reported that vitiligo-like lesions which appeared in the patients receiving PD-1 inhibitors were clinically and biologically distinct from vitiligo (9). Vitiligo-like lesions localized on photoexposed areas with a specific depigmentation pattern without Koebner phenomenon, and were not associated with any personal or family histories of vitiligo, thyroiditis, or other autoimmune disorders. These clinical characteristics are in good agreement with those in our case. But, in our case, skin depigmentation started from photoexposed area and gradually spread throughout the whole body including a trunk in the end. Rashighi et al. reported that gene expression in depigmented skin from vitiligo patients revealed an IFN-γ-specific signature, including elevated expression of chemokine CXCL10 (10). CXCL10 levels were elevated in both skin and serum in the vitiligo patients, and CXCR3, its receptor, was expressed on CD8+ T cells (10-12). They also reported that the perilesional skin of vitiligo-like lesions in melanoma patients treated with nivolumab was characterized by an extensive infiltrate of CXCR3 expressing CD8+ T cells. CXCR3 was also expressed on CD8+ T cells in our case (Figure 3).
It is unclear at present why some patients develop immune-related vitiligo-like depigmentation and the others not. Our patient had a hobby of gardening, and enjoyed it without wearing sunscreen in midsummer daytime when nivolumab treatment was continued. We think that antigens released by the destruction of melanocytes due to strong sunburn might have triggered the development of his vitiligo-like lesions. Alternatively, his NSCLC tumors might simply have carried a common antigen with melanocytes. Uenami et al. reported the first case of vitiligo-like lesion associated nivolumab in a patient with lung adenocarcinoma in 2017 (13). We should also take it into account that an additional trigger such as excessive sunburn might induce skin toxicity during anti-PD-1 treatment in patients with NSCLC.
Conclusions
We experienced a rare case of metastatic NSCLC who developed vitiligo-like lesions during nivolumab treatment. Excessive sunburn during anti-PD-1 treatment might have been a trigger an immune related dermatologic toxicity.
Acknowledgements
We thank the patient and family who contributed to this report and Yachiyo Kumamoto for her contribution to the immunofluorescent experiments.
Footnote
Conflicts of Interest: Dr. Isei T and Dr. Imamura F report personal fees from Ono Pharmaceutical CO. LTD. and Bristol-Myers Squibb, outside the submitted work. Other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Speeckaert R, van Geel N. Vitiligo: An Update on Pathophysiology and Treatment Options. Am J Clin Dermatol 2017;18:733-44. [Crossref] [PubMed]
- Manga P, Elbuluk N, Orlow SJ. Recent advances in understanding vitiligo. F1000Res 2016.5. [PubMed]
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol 2017;44:117-22. [Crossref] [PubMed]
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol 2017;177:708-18. [Crossref] [PubMed]
- Larsabal M, Marti A, Jacquemin C, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol 2017;76:863-70. [Crossref] [PubMed]
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med 2014;6. [Crossref] [PubMed]
- Kaur M, Bagga PK, Kaur T, et al. Evaluation of Histologically and Histochemically Proven Cases of Vitiligo and its Correlation with CD4+ and CD8+ Lymphocyte Counts using Flow Cytometry. J Clin Diagn Res 2017;11:EC09-EC12. [PubMed]
- Wang XX, Wang QQ, Wu JQ, et al. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol 2016;174:1318-26. [Crossref] [PubMed]
- Uenami T, Hosono Y, Ishijima M, et al. Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: A case report. Lung Cancer 2017;109:42-4. [Crossref] [PubMed]


