Lung transplantation, ex-vivo reconditioning and regeneration: state of the art and perspectives
Introduction
Lung transplantation is a consolidated surgical therapy of end-stage pulmonary failure, in selected patients, when maximal medical therapy fails. The shortage of donor pool available for transplantation is often not enough to satisfy the request. It is estimated that only 15–20% of the multi-organ donors have suitable lungs for transplantation (1,2). This situation leads to an increasing waiting list mortality, condition seen as failure of a lung transplant program.
Right now there are several strategies described to overcome the donor pool shortage, one of them is the possibility to recondition lung graft with an “ex-vivo” technique, called ex-vivo lung perfusion (EVLP), allowing evaluation and assessment, reconditioning and eventually the final availability of organs not otherwise suitable for transplantation. The EVLP is nowadays a clinical reality and has several destinations of use, from the evaluation of lungs not otherwise evaluable (i.e., ECMO donors), to the recondition of wet lungs affected by pulmonary (usually neurogenic) edema, and finally the possibility to evaluate lung function in donors from cardiac death (DCD), both in controlled (cDCD) and uncontrolled (uDCD) donors.
Future applications of EVLP can be the improving of the technique already in use, the implementation of number of lungs evaluated and regenerated by EVLP and improving the use of portable device of lung perfusion [i.e., Organ Care System (OCS)]. In addition, cell therapy could have a great potential in conditioning ex-vivo lungs for transplantation.
The aim of this article was to get an overview of the state of the art and the future perspectives of EVLP, including cell therapy, after a non-systematic, narrative review of the literature and analyzing and retracing our personal experience in this field.
Methods
To analyze the state of the art and the potentials of EVLP and regenerative medicine in the field of lung transplantation, the authors made a non-systematic, narrative review of the literature. The primary source used was MEDLINE, using the following strings search:
- (“Lung Transplantation”[Majr]) AND (“Stem Cells”[Mesh] OR “Stem Cell Transplantation”[Mesh] OR “Cell Transplantation”[Mesh] OR “Regenerative Medicine”[Mesh] OR “Regeneration”[Mesh] OR “Cell- and Tissue-Based Therapy”[Mesh]);
- (((“Lung Transplantation”[Majr]) AND “Perfusion”[Mesh])) AND ((EVLP) OR ex-vivo);
- Manual selection of paper by authors.
In addition, we analyze the technical aspect, including surgical skills, of the EVLP procedure, looking at our personal experience compared with the protocols of other high-volume centers.
Finally, we made a technical and descriptive point of view of what is the actual scenario regarding cell therapy and regenerative medicine, once more looking at our personal experience first, and compared with the literature and what is going on.
EVLP
EVLP is a technique capable to assess, recondition and prolong the preservation of grafts otherwise considered marginal or not evaluable for transplantation (3,4).
Steen and colleagues in 2001 successfully developed and applied for the first time EVLP to evaluate the lungs of non-heart beating donor (Lund protocol) (3,5).
Seven years later a different EVLP protocol was proposed by Cypel and coworkers (Toronto protocol) (6). Nowadays many research groups are involved in further developments of EVLP to ensure a greater number of lungs suitable for transplantation and to reduce the mortality of patients on the waiting list.
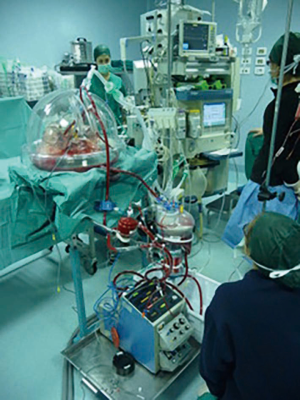
There are different commercialized devices of EVLP system, but the basic circuit includes a ventilator, an endotracheal tube, a lungs chamber, a blood reservoir, a blood pump, a heater, a membrane lung, a leukocyte filter, and a cannula to connect EVLP circuit to the pulmonary artery (Figure 1). The Steen™ solution is the most used perfusion solution with an optimal osmolarity, high dextran content and antioxidative property such as to ensure a protection of the vascular endothelium from macrophage and leukocyte activation as well as other injuries (7). Regardless of the applied protocol, the perfusate (commonly the SteenTM solution) is drained from the left atrium, it is deoxygenated and enriched in carbon dioxide by the membrane lung, and is pumped into the ventilated lungs through the pulmonary artery. The heater exchanger slowly heats the graft at the beginning of the procedure and then maintains the perfusate temperature at 37 °C. Thus, contrarily to the classic static cold storage (4 °C), still the gold standard of grafts preservation, EVLP keeps the lungs at physiological temperatures allowing therefore to evaluate the lung function.
Besides the Toronto and Lund EVLP protocols, a more recent transportable EVLP device is now available, OCS™. In both the Toronto and Lund protocols, the graft, before the EVLP, is exposed to a period of static cold storage mainly required to transport the lungs from the donor to the recipient hospital. The EVLP protocols differ in many technical aspects: left atrium state (open in the Lund Protocol), and perfusate composition (acellular SteenTM solution in the Toronto Protocol vs. cellular with hematocrit of 15% in the Lund one). In the Lund Protocol the pulmonary artery pressure (PAP) is maintained up to 20 mmHg, the perfusion starts at 15 °C, and the ventilation is minute ventilation is gradually increased up to 100 mL/kg/min and respiratory frequency up to 15–20 per min. respiratory rate (RR) of 7 bpm, positive end-expiratory pressure (PEEP) of 5 cmH2O and fraction of inspired oxygen (FiO2) of 21%. In the Toronto technique the PAP is 10–15 mmHg, the left atrial pressure is 3–5 mmHg, the perfusion starts at 25 °C, and tidal volume (Vt) is set to 7 mL/kg, respiratory rate to 7 bpm, PEEP to 5 cmH2O and FiO2 to 21%.
The OCS™ (8) is a portable system that ensure perfusion, ventilation, and monitoring of lungs at physiological temperature also during the transportation, thus the cold ischemia time is extremely shortened.
Regardless of the applied protocol, EVLP aims to increase the number and, eventually, to improve the outcome, of lungs transplantation. During EVLP the donor lungs can be evaluated but also reconditioned. Several therapeutic approaches are currently under investigation. Successes have been reported administering drugs, such as antibiotics, fibrinolytics, and vasodilators, all of which could be administered at high concentration without the risk of systemic toxicity.
Positive bronchoalveolar lavage of the donor lungs increases the risk of developing post-transplant pneumonia (9,10). The risk of developing ventilator-associated pneumonia is also increased by possible prolonged endotracheal intubation and mechanical ventilation following lung transplantation. It has been demonstrated that administration of broad-spectrum antibiotics to infected grafts during EVLP can reduce the bacterial burden and the inflammatory reaction leading to possible transplantation of otherwise not suitable grafts (11-13).
The ischemic-reperfusion injury (IRI), a pathological process occurring when the blood flow is absent and subsequently restored, has a pivotal role in the development of post-transplant complications, such as primary grafts dysfunction (PGD) or chronic lung allograft dysfunction (CLAD). Indeed, the restoration of blood flow leads to endothelial dysfunction, reactive oxygen species production, activation of the coagulation and immune system, with release of pro-inflammatory cytokines and chemokines, and cell death via necrosis and apoptosis. An over production of pro- and anti-inflammatory interleukins and tumor necrosis factor-alpha has been recorded during EVLP and it has been positive correlated with post-transplant complication (14). Subsequently, attenuation of the ischemic-reperfusion injury has been attempted through administration of steroids (15), N-acetyl cysteine (16) or direct removal of the cytokines by adsorbent membrane (17) or filters (18).
Lung reconditioning through gene therapy has been evaluated by administration of adenoviral vector encoding human interleukin-10 (19). This cytokine inhibits the pro-inflammatory cytokines secretion inactivating the antigen-presenting cells of the immune system.
Fibrinolytic treatment has been also tested in swine model. The success of this strategy appears extremely important with DCD donors, when timely heparin use might not be possible. Urokinase administration during EVLP reduced pulmonary vascular resistance and improved oxygenation in a pre-clinical DCD model (20). Conversely, the infusion of alteplase, also in a DCD model, did not show any improvements in gas exchange or pulmonary vascular resistance compared to conventional treatment (21). Fibrinolytic treatment was also successfully applied to recondition human donor lungs with acute pulmonary embolism, which is normally considered a contraindication to lungs donation (22).
It has been demonstrated that the administration of β2-adrenoreceptor and adenosine A2A receptor agonists attenuates IRI. In a canine DCD model, the β2-adrenoreceptor agonist inhalation during the period before procurement of the lungs (23) and during EVLP (24) showed a protective effect. β2-agonist administration improved the pulmonary function, lowering peak airway pressure, pulmonary arterial pressure, pulmonary vascular resistance and increasing lung compliance. Moreover, some studies have demonstrated that β2-agonists enhance the alveolar fluid clearance through the upregulation of the cystic fibrosis transmembrane conductance regulator (CFTR), thus decreasing acute lungs injury. β2-agonist administration during EVLP (24) increased CFTR expression and seems to attenuate grafts injury via upregulation of alveolar fluid clearance.
An improvement of PaO2/FiO2 is also reported in a porcine DCD model following administrating adenosine A2A receptor agonist during EVLP, preceded both by short- (25) and long-term (26) preservation at 4 °C.
Currently, lungs injured by aspiration of gastric content are not considered for transplant. Conventional EVLP seems ineffective in improving lungs with an ab ingestis damage (27), however direct instillation of exogenous surfactant via a bronchoscope (28) during EVLP showed promising results.
Lungs ventilation with hydrogen during EVLP showed anti-inflammatory, antiapoptotic, and antioxidative properties (29). Other gases, such as carbon monoxide (30), nitric oxide (31) and hydrogen sulfide (32) showed positive results whereas argon and xenon did not seem to improve lungs functions (33).
Stem cell therapy is an extremely promising technique for lungs transplantation. Mesenchymal stem cells (MSCs) (34) and multipotent adult progenitor cells (35) are able to regenerate the damaged tissue with new cells and secrete paracrine factors that regulate epithelial and endothelial permeability, thus enhancing alveolar fluid clearance and attenuating immune response in injured lungs.
In conclusion, EVLP proved to be an effective procedure to evaluate and recondition lungs prior to transplantation. Improved EVLP technical aspects and promising therapeutic opportunities might further increase the number of lungs available for transplants thus further reduce the mortality on waiting list.
Surgical notes
The surgical point of view about using EVLP is mainly relate to the donor surgeon. In fact, the decision to evaluate the lungs with EVLP, even if is previously scheduled, is taken at the donor site, after visual evaluation and manual inspection of lungs, analysis of arterial blood gas (ABG) test during the brain death period, excluding pneumonia or major purulent bronchial secretions and so on.
Technically the retrieval of lungs follows the standard fashion, apart from the necessity to have more trachea for easily and handily cannulation ex vivo of lung isolated by double lumen endobronchial tube. If possible, with regard to the concomitant cardiectomy done by heart surgeon, it is desirable to obtain the longest possible pulmonary artery (PA), to allow, once more, easily and handily cannulation of PA for ex-vivo perfusion. If an enough main pulmonary artery cannot be reached, we usually resect a vascular graft (segment of descending aorta), which is end-to-end anastomosed to the main PA. After anterograde (through PA) pneumoplegia and retrograde (through pulmonary veins) perfusion with specific solutions (i.e., Perfadex®) and graft retrievals, the lungs (not divided) are cool stored in ice and transported to the recipient site, where EVLP is ready to start. Once arrived at recipient site the lungs are connected to the machine perfusion by in cannulation of the PA and eventually, if used a “close atrium technique”, by an end-to-end anastomosis between left atrial cuff and venting cannula connected to the reservoir chamber. If it is used the “open atrium technique”, the venting from the left atrium is left free in the chamber and collected by gravity in the reservoir chamber. A temperature probe is placed in the area of left atrial cuff. Once cannulations are correctly performed, EVLP can start to run. Following a specific perfusion/ventilation protocol, the flow of perfusion is incremented slowly and the ventilation started once the desired temperature has been reached. During EVLP multiple examinations are carried out, even selectively from each pulmonary vein, to assess the function of lungs and lobes separately. This type of selective evaluation, along with a visual and bronchoscopic assessment, can bring to the final decision to accept the lungs for transplantation definitely, or in alternative after downsizing of part of the lungs (even by lobectomies), which it can be performed during the EVLP run itself (36). The decision to downsize the graft can be taken for size mismatch (if grafts are oversized in comparison to the recipient chest) or because of the presence of damages in specific areas (37).
At the end of the EVLP procedure and eventual downsizing, the lungs are divided and re-cooled down separately, ready to be used for transplantation.
Cell therapy and lung transplantation
Among the stem cell types investigated to improve lung transplantation outcome, MSCs gained a prominent role (38), in reason of their immunomodulatory and regenerative properties (39) by which tolerance to the transplanted organ is sought, mainly relying on upregulation of T-reg immune response (40). Such cell therapy approach is particularly interesting also with regards to the refinement or reduction of the use of immunosuppressants.
With regards to pre-clinical studies, MSCs were successfully used to reduce ischemia/reperfusion (I/R) injury in a pig animal model (41). MSCs were administered both in the pulmonary artery or nebulized endobronchial, with the latter showing full restoration of pulmonary-vascular-resistance and oxygenation. Thus, it is possible to envision the use of recipient-derived MSCs to pretreat non-heart-beating lung donors before organ retrieval to improve the post-ischemic allograft function. Another study investigated a possible synergistic effect of MSC engraftment with ischemia post-conditioning (IPO) in rats. Intriguingly, IPO pretreatment enhanced the MSC survival, overall engraftment and protective effect (42). Furthermore, also cold I/R injury was addressed in a murine orthotopic lung transplantation model. Intravenously delivered MSCs significantly improved graft arterial oxygenation capacity and promoted an anti-inflammatory microenvironment (43). Taken together, these results stress the concept that the choices concerning the route and appropriate time of MSC administration are crucial to obtain optimal cell therapy outcomes.
Recently, the possibility to improve MSC regenerative properties by gene therapy approaches lead to promising outcomes. In detail, viral interleukin 10 (IL10)-engineered MSCs were tested in rats for lung I/R injury prevention, in reason of IL10 anti-inflammatory and anti-apoptotic activity (44). The genetically-modified MSCs modulated successfully many parameters of damage, including mean blood oxygenation, microvascular permeability, wet-to-dry weight ratios, apoptosis and inflammatory response (45). Analogously, hepatocyte growth factor (HGF)-modified MSCs showed similar beneficial effects compared to unmodified MSCs, mainly due to HGF anti-apoptotic activity (46). Finally, preliminary results were obtained also in a human ex vivo reperfusion model. To this aim, lungs discarded for transplantation and subjected to prolonged ischemic time were used. Briefly, the authors determined that intravenous administration of 5×106 clinical-grade human (h)MSCs restored alveolar fluid clearance to normal levels. Interestingly, this effect was reduced by intrabronchial administration of a neutralizing antibody to keratinocyte growth factor (47).
Up to date, the clinical studies addressing MSC-based cell therapy approaches in lung transplant settings gained crucial insights on their potential therapeutic use. Safety and feasibility of hMSCs administration in CLAD were successfully investigated by a phase I study (ClinicalTrial.gov; NCT01175655). Briefly, 2×106 per kilogram hMSCs were infused via a peripheral vein in bronchiolitis obliterans patients twice a week for 2 weeks, with a 52 weeks follow-up (48). The treatment was well-tolerated by the patients (n=10) without any serious adverse event. Other studies, whose results are still not published, proposed to evaluate safety and feasibility of allogeneic (ClinicalTrial.gov; NCT02181712) or autologous (ClinicalTrial.gov; NCT01668576) hMSCs to tackle with bronchiolitis obliterans. More importantly, a phase II interventional multi-center randomized study (ClinicalTrials.gov; NCT02709343) started on April 2017 will evaluate the efficacy of hMSC treatment in lung transplant patients (n=82), with estimated completion on June 2022.
Regarding novel therapeutic strategies, a more innovative cell-free approach has been recently proposed, following the increasing evidences that extracellular vesicles (EVs) secreted by MSCs (MSC-EVs) recapitulate the regenerative properties of parental MSCs. EVs represent a broad class of membrane-enclosed cytoplasm-containing bodies of cell origin, which can be divided mainly into two categories with potentially different functionalities: exosomes, generated by the endosomal pathway, and microvesicles, produced by shedding of the plasma membrane (49). EVs are currently investigated as cell-to-cell communication tool (44), as biomarkers of disease (50), and also as therapeutic agents (51). Indeed, EVs are loaded with bioactive molecules resembling the content of the cells of origin, which are released inside target cells or tissues upon fusion of EVs with the plasma membrane.
MSC-EVs have been successfully tested as an alternative to hMSC administration in an ex vivo lung perfusion model (52). In detail, MSC-EV treatment increased alveolar fluid clearance in a dose-dependent manner and improved airway and hemodynamic parameters. Intriguingly, co-administration of MSC-EVs with anti-CD44 antibody reduced these effects, suggesting that CD44 is an important mediator for internalization of MSC-EVs inside target cells. Importantly, the administration of EVs derived from a non-stem source such as lung fibroblasts had no whatsoever effect.
EVs found in bronchoalveolar lavage fluids seem to be useful also as prognostic or diagnostic biomarkers of lung transplant outcome. For instance, levels of EVs originated from epithelial and red blood cells were shown to be significantly upregulated in patients suffering from CLAD. Moreover, higher levels of epithelial cell EVs were associated with shorter overall survival, whereas higher levels of red blood cell EVs were associated with worse disease-specific survival (53). EVs were also studied as biomarkers in the context of acute cellular rejection. The bronchoalveolar fluid was retrieved after lung transplant and the EV RNA content analyzed. An intense inflammatory profile was detected, skewed toward both innate and adaptive immune responses (54).
Discussion
Besides different protocols and techniques existing at the moment, EVLP is a platform capable of increasing the number and, hopefully, improving the outcome, of lung transplantation. Donor lungs can be evaluated and reconditioned during EVLP. Positive results have been reported administering drugs, such as antibiotics, fibrinolytics, and vasodilators, during EVLP. Several therapeutic options are currently under investigation and some trials are ongoing. Eventually EVLP would play a role in restoring tissue integrity after ischemic injury. One of the promising destination of use of the EVLP platform could be the potential role as a tool to repair ischemic-injury damage, that ever occurs during transplantation.
Successes have been reported administering drugs, such as antibiotics, fibrinolytics, and vasodilators, using in this way the EVLP with a curative approach. Further studies are needed to consolidate and confirm the therapeutic role of the ex-vivo procedure. EVLP might improve grafts function and prevent the immune response of the recipient directed towards the antigens of the damage cells, thus decreasing of incidence of primary graft dysfunction as well as chronic rejection. Furthermore, EVLP will increase the overall number of grafts available for transplantation, overcoming the organs shortage, that still remain one of the greatest obstacles in the clinical practice.
From the cell therapy point of view, several preclinical and clinical studies are currently ongoing, evaluating new kind of EVLP models that can reduce the signs of cellular death. In particular, the possibility to reach the damaged cells with MSCs open new perspectives of cell’s regeneration. Waiting for new organs completely compatible made from scaffolds, a possible strategy to improve the outcome of lung transplantation could be the graft tissue regeneration and the immunomodulation of the recipient performed using MSCs. The encouraging results obtained in some lung perfusion models addressing MSC-based cell therapy approaches, show that this is the right way forward.
Nowadays, the EVLP platform, both static and portable (OCSTM) is a consolidated tool to preserve and assess donor lungs, both for graft from brain death donors (DBD) and from controlled or uncontrolled cardiac death donors.
The role of EVLP in reconditioning injured lungs, such as due to contusions or infections, as well as in modulating the recipient immune response after transplantation, is still under investigation in both preclinical and clinical studies. Although the achievement of an optimized procedure is still far away, the preliminary results are encouraging, and the future prospective seems to be quite promising.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Snell GI, Griffiths A, Levvey BJ, et al. Availability of lungs for transplantation: Exploring the real potential of the donor pool. J Heart Lung Transplant 2008;27:662-7. [Crossref] [PubMed]
- Roman MA, Nair S, Tsui S, et al. Ex vivo lung perfusion: a comprehensive review of the development and exploration of future trends. Transplantation 2013;96:509-18. [Crossref] [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431. [Crossref] [PubMed]
- Steen S, Liao Q, Wierup PN, et al. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg 2003;76:244. [Crossref] [PubMed]
- Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319-25. [Crossref] [PubMed]
- Carnevale R, Biondi-Zoccai G, Peruzzi M, et al. New insights into the Steen solution properties: breakthrough in antioxidant effects via NOX2 downregulation. Oxid Med Cell Longev 2014;2014. [Crossref] [PubMed]
- Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet 2012;380:1851-8. [Crossref] [PubMed]
- Ruiz I, Gavalda J, Monforte V, et al. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant 2006;6:178-82. [Crossref] [PubMed]
- Avlonitis VS, Krause A, Luzzi L, et al. Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur J Cardiothorac Surg 2003;24:601-7. [Crossref] [PubMed]
- Karamanou DM, Perry J, Walden HR, et al. The effect of ex-vivo perfusion on the microbiological profile of the donor lung. J Heart Lung Transplant 2010;29:S94. [Crossref]
- Andreasson A, Karamanou DM, Perry JD, et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Heart Lung Transplant 2014;33:910-6. [Crossref] [PubMed]
- Nakajima D, Cypel M, Bonato R, et al. Ex vivo perfusion treatment of infection in human donor lungs. Am J Transplant 2016;16:1229-37. [Crossref] [PubMed]
- Mohamed MS. Translational Insights on Lung Transplantation: Learning from Immunology. Iran J Immunol 2015;12:156-65. [PubMed]
- Martens A, Boada M, Vanaudenaerde BM, et al. Steroids can reduce warm ischemic reperfusion injury in a porcine donation after circulatory death model with ex vivo lung perfusion evaluation. Transpl Int 2016;29:1237-46. [Crossref] [PubMed]
- Geudens N, Wuyts WA, Rega FR, et al. N-acetyl cysteine attenuates the inflammatory response in warm ischemic pig lungs. J Surg Res 2008;146:177-83. [Crossref] [PubMed]
- Kakishita T, Oto T, Hori S, et al. Suppression of inflammatory cytokines during ex vivo lung perfusion with an adsorbent membrane. Ann Thorac Surg 2010;89:1773-9. [Crossref] [PubMed]
- Iskender I, Cosgun T, Arni S, et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J Heart Lung Transplant 2017. [Epub ahead of print]. [PubMed]
- Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 2009;1:4ra9. [Crossref] [PubMed]
- Inci I, Zhai W, Arni S, et al. Fibrinolytic treatment improves the quality of lungs retrieved from non-heart-beating donors. J Heart Lung Transplant 2007;26:1054-60. [Crossref] [PubMed]
- Liersch-Nordqvist A, Fakhro M, Pierre L, et al. The impact of alteplase on pulmonary graft function in donation after circulatory death: An experimental study. Annals of Medicine and Surgery 2017;22:1-6. [Crossref] [PubMed]
- Inci I, Yamada Y, Hillinger S, et al. Successful lung transplantation after donor lung reconditioning with urokinase in ex vivo lung perfusion system. Ann Thorac Surg 2014;98:1837-8. [Crossref] [PubMed]
- Sakamoto J, Chen F, Nakajima D, et al. The effect of β-2 adrenoreceptor agonist inhalation on lungs donated after cardiac death in a canine lung transplantation model. J Heart Lung Transplant 2012;31:773-9. [Crossref] [PubMed]
- Kondo T, Chen F, Ohsumi A, et al. Beta2-adrenoreceptor agonist inhalation during ex vivo lung perfusion attenuates lung injury. Ann Thorac Surg 2015;100:480-6. [Crossref] [PubMed]
- Mulloy DP, Stone MS, Crosby IK, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg 2012;144:1208-15. [Crossref] [PubMed]
- Wagner CE, Pope NH, Charles EJ, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg 2016;151:538-45. [Crossref] [PubMed]
- Khalifé-Hocquemiller T, Sage E, Dorfmuller P, et al. Ex vivo perfusion worsened lung injuries induced by gastric acid aspiration in pigs. J Heart lung Transplant 2011;30:S135. [Crossref]
- Khalifé-Hocquemiller T, Sage E, Dorfmuller P, et al. Exogenous surfactant attenuates lung injury from gastric-acid aspiration during ex vivo reconditioning in pigs. Transplantation 2014;97:413-8. [Crossref] [PubMed]
- Haam S, Lee S, Paik HC, et al. The effects of hydrogen gas inhalation during ex vivo lung perfusion on donor lungs obtained after cardiac death. Eur J Cardiothorac Surg 2015;48:542-7. [Crossref] [PubMed]
- Dong B, Stewart PW, Egan TM. Postmortem and ex vivo carbon monoxide ventilation reduces injury in rat lungs transplanted from non-heart-beating donors. J Thorac Cardiovasc Surg 2013;146:429-36.e1. [Crossref] [PubMed]
- Dong BM, Abano JB, Egan TM. Nitric oxide ventilation of rat lungs from non-heart-beating donors improves posttransplant function. Am J Transplant 2009;9:2707-15. [Crossref] [PubMed]
- George TJ, Arnaoutakis GJ, Beaty CA, et al. Inhaled hydrogen sulfide improves graft function in an experimental model of lung transplantation. J Surg Res 2012;178:593-600. [Crossref] [PubMed]
- Martens A, Montoli M, Faggi G, et al. Argon and xenon ventilation during prolonged ex vivo lung perfusion. J Surg Res 2016;201:44-52. [Crossref] [PubMed]
- Gennai S, Monsel A, Hao Q, et al. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant 2015;15:2404-12. [Crossref] [PubMed]
- La Francesca S, Ting AE, Sakamoto J, et al. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: an initial pilot and feasibility study. Transplant Res 2014;3:19. [Crossref] [PubMed]
- Nosotti M, Rosso L, Mendogni P, et al. Graft downsizing during ex vivo lung perfusion: case report and technical notes. Transplant Proc 2014;46:2354-6. [Crossref] [PubMed]
- Mendogni P, Palleschi A, Tosi D, et al. Lobar Lung Transplantation From Deceased Donor: Monocentric Experience. Transplant Proc 2017;49:682-5. [Crossref] [PubMed]
- Mohamed MS. Mesenchymal Stem Cells and Lung Transplantation: A Couple for a Perfect Relationship. Journal of stem cells 2015;10:63-7. [PubMed]
- Ho MS, Mei SH, Stewart DJ. The Immunomodulatory and Therapeutic Effects of Mesenchymal Stromal Cells for Acute Lung Injury and Sepsis. J Cell Physiol 2015;230:2606-17. [Crossref] [PubMed]
- Guo K, Ikehara S, Meng X. Mesenchymal stem cells for inducing tolerance in organ transplantation. Front Cell Dev Biol 2014;2:8. [Crossref] [PubMed]
- Wittwer T, Rahmanian P, Choi YH, et al. Mesenchymal stem cell pretreatment of non-heart-beating-donors in experimental lung transplantation. J Cardiothorac Surg 2014;9:151. [Crossref] [PubMed]
- Chen S, Chen L, Wu X, et al. Ischemia postconditioning and mesenchymal stem cells engraftment synergistically attenuate ischemia reperfusion-induced lung injury in rats. J Surg Res 2012;178:81-91. [Crossref] [PubMed]
- Tian W, Liu Y, Zhang B, et al. Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice. Lung 2015;193:85-95. [Crossref] [PubMed]
- Ragni E, Banfi F, Barilani M, et al. Extracellular Vesicle-Shuttled mRNA in Mesenchymal Stem Cell Communication. Stem cells (Dayton, Ohio) 2017;35:1093-105. [Crossref] [PubMed]
- Manning E, Pham S, Li S, et al. Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther 2010;21:713-27. [Crossref] [PubMed]
- Chen S, Chen X, Wu X, et al. Hepatocyte growth factor-modified mesenchymal stem cells improve ischemia/reperfusion-induced acute lung injury in rats. Gene Ther 2017;24:3-11. [Crossref] [PubMed]
- McAuley DF, Curley GF, Hamid UI, et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol 2014;306:L809-15. [Crossref] [PubMed]
- Chambers DC, Enever D, Lawrence S, et al. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl Med 2017;6:1152-7. [Crossref] [PubMed]
- Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066. [Crossref] [PubMed]
- Gonzalez E, Falcon-Perez JM. Cell-derived extracellular vesicles as a platform to identify low-invasive disease biomarkers. Expert Rev Mol Diagn 2015;15:907-23. [Crossref] [PubMed]
- Gyorgy B, Hung ME, Breakefield XO, et al. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol 2015;55:439-64. [Crossref] [PubMed]
- Gennai S, Monsel A, Hao Q, et al. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am J Transplant 2015;15:2404-12. [Crossref] [PubMed]
- Harms A, Fuehner T, Warnecke G, et al. Epithelial and Erythrocyte Microvesicles From Bronchoalveolar Lavage Fluid Are Elevated and Associated With Outcome in Chronic Lung Allograft Dysfunction. Transplantation 2015;99:2394-400. [Crossref] [PubMed]
- Gregson AL, Hoji A, Injean P, et al. Altered Exosomal RNA Profiles in Bronchoalveolar Lavage from Lung Transplants with Acute Rejection. Am J Respir Crit Care Med 2015;192:1490-503. [Crossref] [PubMed]