Pathologic complete response after induction therapy—the role of surgery in stage IIIA/B locally advanced non-small cell lung cancer
Introduction
Pathologic complete response (pCR), defined as the absence of tumor cells in all specimens (ypT0N0) is an important prognostic factor in the management of locally advanced non-small cell lung cancer (NSCLC). In various studies pCR determined a long-term survival (LTS), was associated with lower incidence of local and distant recurrence, as well as resulted in favorable progressive-free survival (PFS) (1). Similar prognostic significance of pCR has been reported in esophageal, rectal, breast and bladder cancer (2-6). Irrespective its prognostic significance, the implication of pCR in daily practice is limited, due to inaccurate non-operative evaluation of the tumor response, wide pCR variability depending on treatment protocol and therefore, unpredictable incidence of pCR (7). In addition, despite the robust association between favorable survival and pCR, the correlation between various clinical and pathological factors has rarely been analyzed (1,8).
The aim of our retrospective study was to assess the correlation between clinical and pathological factors vs. pCR and to analyze a LTS, PFS and the tumor recurrence pattern in the postoperative course.
Methods
A cohort of patients with locally advanced NSCLC in stage IIIA/B treated with IT and subsequent surgery at single center was retrospectively reviewed. A subgroup of patients with pCR was extracted for further analysis. Pre-treatment staging was based on the computed tomography (CT), 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), and cranial magnetic resonance imaging (MRI). The PET-positive mediastinal lymph nodes were further investigated with fine-needle transbronchial biopsy and/or via cervical video mediastinoscopy. Pre-surgical re-staging included CT and/or FDG-PET in order to exclude distant metastasis and extensive mediastinal lymph node involvement. The course of neo-adjuvant chemoradiotherapy (CRT) was standardised and included platinum-based chemotherapy (cisplatin 20 mg/m2/day on day 1–5 in week 1 and 5, and etoposide 90 mg/m2/day on day 3 in week 1 and 5) with concomitant high-dose radiation of up to 50.4 Gy applied to the primary lesion and to the mediastinal lymph nodes. The course of neo-adjuvant chemotherapy (CHT) was also platinum-based. Patient selection for surgery after IT was in accordance with the response evaluation criteria in solid tumors (RECIST) and took place within the multidisciplinary conference (9). Only patients with radiological complete/partial regression and stable disease were offered surgery within 6–8 weeks after completing the IT. In patients with progressive disease, unresectable T4-tumor and pathologically proven N3-stage or with severe reduced cardiopulmonary status, the surgery was denied. At least lobectomy with pathologically proven complete resection on the bronchial stump margin and pulmonary vessels (R0) were defined oncological adequate. The lymph node dissection included all ipsilateral mediastinal lymph nodes, irrespective the tumor location.
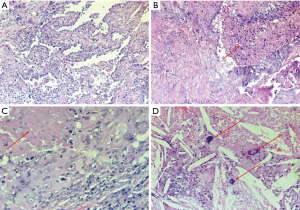
The pathological workup of the specimens was performed according to the Junker classification (10). Thus, the pathologic response was defined “complete” by the absence of viable cancer cells in the primary lesion and within the mediastinal lymph nodes (ypT0N0). Tumors with the micro-foci of malignancy were considered residual lesions and were excluded from further analysis (Figure 1). The preoperative clinical data, patient characteristics including the clinical, pathologic tumor description, and surgical features were collected. Tumor response to the induction therapy (IT), extent of surgery, completeness of resection, number of dissected lymph nodes, perioperative morbidity and mortality, postoperative survival (POS) and PFS rate, local (bronchial stump), loco-regional (ipsilateral pulmonary and mediastinal lymph nodes) and distant (other organs or contralateral pulmonary) recurrence as well as tumor-related deaths were subjects of further analysis. LTS was defined as a survival of more than 36 months. The clinical and pathological tumor staging was based on the eighth edition of the TNM classification for NSCLC (11).
The statistical analysis was performed using SPSS (Version 21, IBM, USA) and stratified by descriptive statistics, Chi-square correlation analysis, Kaplan-Meier survival curves and estimated 3- and 5-year survival combined with long-rank tests and Cox multivariate-analysis. For all tests, P value of <0.05 was considered statistically significant.
Results
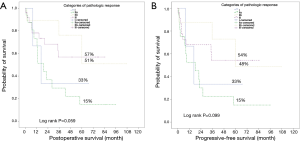
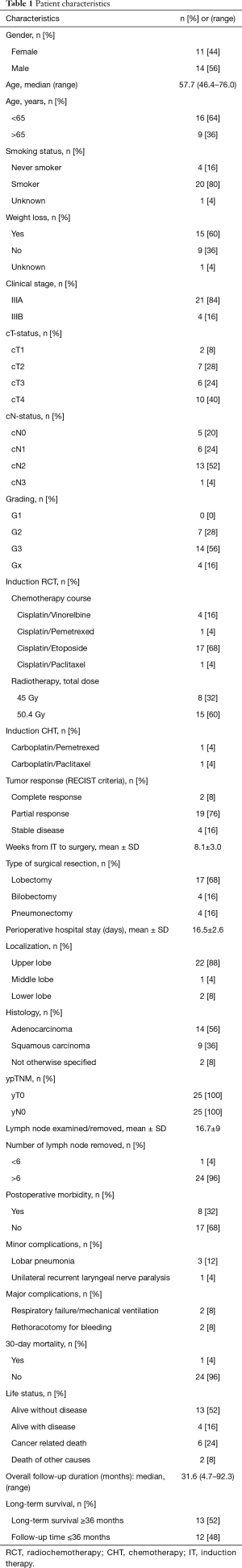
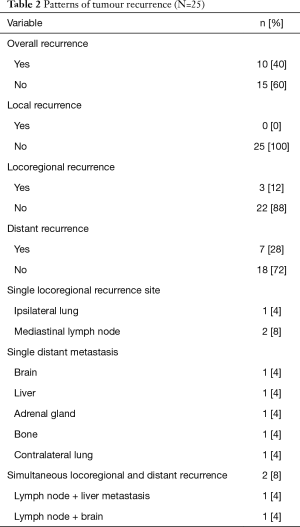
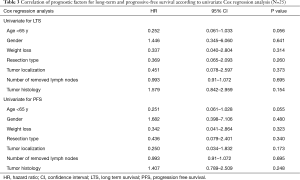
Between March 2008 and January 2017, a total of 54 patients underwent curative pulmonary resections following the IT. Based on estimated POS rate and progressive-free interval pCR was associated with favorable survival rate and recurrence-free interval compared to other pathologic response categories. The estimated 3- and 5-year LTS rates for pCR were 63% and 57%, respectively. The estimated 3- and 5-year PFS rates for pCR were 61% and 54%, respectively. The median POS and PFS in patients with pCR were not reached (Figure 2). Based on those findings, 25 patients (46.3%) with pCR were selected for further analysis. The patient characteristics are given in the Table 1. Twenty-three patients underwent a neo-adjuvant RCT as IT, whereas the neo-adjuvant CHT as IT course was performed in 2 patients. According to the RECIST criteria the partial response was present in most of our patients without a robust correlation to the pathologic findings. The mean length of perioperative hospital stay was 16.5±2.6 days. The 30-day mortality rate was 4% (n=1) due to postoperative adult respiratory distress syndrome (ARDS) on the 7th day after pneumonectomy. The LTS was noted in 13 patients. The detailed overviews of the patient status at the end of the follow up as well as the patterns of tumor recurrence are presented in the Table 2. During the follow-up the tumor recurrence occurred in 8 patients (32%). In order to assess the correlation of the pCR with different clinical and pathological factors, univariate Cox regression analysis was performed. There was no significant correlation between pCR and those prognostic factors (Table 3).
Full table
Full table
Full table
Discussion
The adequate treatment of the locally advanced NSCLC in stage IIIA/B is a subject of the ongoing multidisciplinary debate. Stage IIIA/B NSCLC is a heterogeneous disease and includes a variable extent of the mediastinal lymph node involvement, ranging from micrometastasis to bulky lymph node disease, usually accompanied with perinodal tumor growth and a various size tumor with a bulky primary lesion invading the neighboring anatomic structures (12,13). The local tumor control and down staging resulting in tumor response and mediastinal lymph node clearance, are potential surrogate endpoints for better patient outcome (7). Particularly, the pCR was proven an independent positive prognostic predictor with superior relevance to mediastinal lymph node clearance, tumor size and gender (8). Robust correlation with favorable survival and tumor control determining the prognostic superiority of pCR in patients with locally advanced NSCLC after IT has been reported in other studies and corresponds with our results (Table 4).

Full table
To date, the estimation of pCR in the preoperative setting is inaccurate. In the published data, the pCR incidence rate is variable, but consistently evident in significant proportion of the patients, differing from 8% to 35% in various IT protocols (1,20-22). Similarly as in our cohort, a high discordance between RECIST criteria and pathologically proven tumor devitalization has been reported (23). Therefore, various radiological features were analysed in order to improve the preoperative pCR prediction. In detail, the CT-based tumor volume change showed significant relationship with pathologic response, as demonstrated by Agrawal et al. (24). In contrast, a lack of correlation between CT-based volume variation and pathologic response was identified by Pöttgen et al. and Cerfolio et al. (25,26). The inconsistence might have resulted from the small sample size, low incidence of pCR that consequently lead to the insufficient correlation of radiological and pathological changes. CT as a staging standard failed to distinguish between viable tumor, necrosis and treatment-associated scarring, based on morphologic changes only. Consequently, the “real” radiologic response cannot be precisely determined by preoperative CT. This would subsequently support the idea of surgical resection for pathologic examination of specimen in order to assess the exact tumor response (27).
The use of FDG-PET for preoperative pCR prediction after IT was investigated in lung, thymic, pancreatic and rectal cancer (28-31). In particular, FDG-PET imaging in patients with locally advanced NSCLC was usually correlated with high rate of false-negative and false-positive findings (32). The relevant difference in predictive value, based on the decrease of standard uptake value, was recently identified in relation to extracerebral distant metastases, without any significant correlation to the local recurrence. However, the influence on the clinical decision making after initial treatment seems to be limited (33). Recent results indicate the utility of standard uptake value and standard uptake ratio in the prediction of pCR (28). Although the PET imaging provides additional metabolic information on the primary tumor and mediastinal lymph nodes, most PET findings require pathological confirmation such as distinguishing between posttreatment changes and tumor response. The potential role of FDG-PET in the restaging is insufficient and needs further investigation (34).
A radiomics model, as an emerging field of quantitative imaging of exact phenotypic tumor information, was also investigated in order to improve the preoperative pCR-prediction. Although a cross validation between pCR and spherical disproportionality of the primary tumor were detected, more radiomics features were found predictive for pathologic gross residual disease. The underlying tumor phenotype seems more relevant for identification of pCR, whereas the conventional features, like tumor volume and size, were more valuable indicators of the IT response (35).
In summary, there are no demographic, radiologic and treatment-related features, which could reliably predict the pCR preoperatively. Even considering the whole spectrum of the available non-invasive and invasive procedures, the re-staging failed to accurately predict the tumor response. The ongoing multidisciplinary debate on a potential role of surgery in locally advanced NSCLC highlights pathologic investigation of a resected specimen as the only accurate identification of the tumor response. This clearly emphasizes a privilege of surgery within multimodality therapy setting in identifying those patients with favorable prognosis and in allowing further stratification of the management, depending on the risk of tumor recurrence. With respect to those findings, the selection process remains in accordance with widely accepted RECIST criteria. However, the interdisciplinary decision making is crucial and strongly dependent on surgical experience within multimodality treatment protocols.
The limitation of our study is its retrospective character as well as the selection bias related to the limited patient number. To our knowledge, no prospectively randomised studies on pCR after IT for locally advanced NSCLC have been published. Therefore, the current multidisciplinary experience is based on retrospective analyses only.
Conclusions
pCR is an important prognostic factor determining favorable survival, but associated with wide variable incidence. No correlations between pCR vs. clinical and pathological factors, including RECIST criteria, could be identified. The surgery as a part of the multimodality therapy seems to provide the accurate identification of tumor response and to allow further patient stratification according to the different risk of tumor recurrence. The interdisciplinary debate on the potential role of surgery is needed to clarify the surgical impact on identification of pCR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was notified by local ethic committee. The approval was not mandatory according to the retrospective data analysis, see statement of ethic committee. Formal consent is not required.
References
- Lococo F, Cesario A, Margaritora S, et al. Long-term results in patients with pathological complete response after induction radiochemotherapy followed by surgery for locally advanced non-small cell lung cancer. Eur J Cardiothorac Surg 2013;43:e71-81. [Crossref] [PubMed]
- Haisley KR, Hart KD, Nabavizadeh N, et al. Neoadjivant chemoradiotherapy with concurrent cisplatin/5-fluoracil is associated with increased pathologic complete response and improved survival compared to carboplatin/paclitaxel in patients with locally advanced esophageal cancer. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Dinaux AM, Amri R, Bordeianou L, et al. The Impact of pathologic complete response in patients with neo-adjuvant treated locally advanced rectal cancer - a large single-center experience. J Gastrointest Surg 2017;21:1153-8. [Crossref] [PubMed]
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. New Eng J Med 2012;366:2438-41. [Crossref] [PubMed]
- Campbell JI, Yau C, Krass P, et al. Comparison of residual cancer burgen, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat 2017;165:181-91. [Crossref] [PubMed]
- Pokuri VK, Syed J, Yang Z, et al. Predictors of complete pathologic response (pT0) to neoadjuvant chemotherapy in muscle –invasive bladder carcinoma. Clin Genitourin Cancer 2016;14:e59-e65. [Crossref] [PubMed]
- Hellmann MD, Chaft J, William W, et al. Pathologic response after neo-adjuvant chemotherapy in resectable non-small cell lung cancers: proposal for the use of “major pathologic response” as a surrogate endpoint. Lancet Oncol 2014;15:e42-e50. [Crossref] [PubMed]
- Pöttgen C, Stuschke M, Graupner B, et al. Prognostic model for long-term survival of locally advanced non-small cell lung cancer patients after neo-adjuvant radiochemotherapy and resection integrating clinical and histopathologic factors. BMC Cancer 2015;15:363. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 1997;123:469-77. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Grouping in the Forthcoming (Eight) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- De Ruysscher D, Vansteenkiste J, Belderbos J, et al. The optimal local treatment of stage IIIA-N2 NSCLC: Is the issue finally settled? J Thorac Oncol 2016;11:284-6. [Crossref] [PubMed]
- Van Meerbeeck JP. The controversial role of surgery in stage III NSCLC. Lancet 2008;9:607-8. [Crossref] [PubMed]
- Friedel G, Buddach W, Dippon J, et al. Phase II Trial of a trimodality regimen for stage III non-small-cell cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol 2010;28:942-8. [Crossref] [PubMed]
- Steger V, Walker T, Mustafi M, et al. Surgery on unfavourable persistent N2-N3 non-small-cell lung cancer after trimodal therapy: do the results justify the risk? Interact Cardiovasc Thorac Surg 2012;15:948-53. [Crossref] [PubMed]
- Isobe K, Hata Y, Sakaguchi S, et al. Pathological response and prognosis of stage III non-small cell lung cancer patients treated with induction chemoradiation. Asia-Pac J Clin Oncol 2012;8:260-6. [Crossref] [PubMed]
- Shumway D, Corbin K, Salga R, et al. Pathologic response rates following definitive dose image-guided chemoradiotherapy and resection for locally advanced non-small cell lung cancer. Lung Cancer 2011;74:446-50. [Crossref] [PubMed]
- Cerfolio RJ, Maniscalco L, Bryant A. The treatment of patients with stage IIIA non-small cell lung cancer from N2 disease: who returns to the surgical arena and who survives. Ann Thorac Surg 2008;86:912-20. [Crossref] [PubMed]
- Kim AW, Liptay MJ, Bonomi P, et al. Neo-adjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg 2011;92:233-41; discussion 241-3. [Crossref] [PubMed]
- Mirimanoff RO. Neoadjuvant chemoradiotherapy followed by surgery for stage IIIa and IIIb non-small-cell lung cancer (NSCLC): is it still justified? Chin Clin Oncol 2015;4:49-57. [PubMed]
- Pöttgen C, Eberhardt W, Stamatis G, et al. Definitive radiochemotherapy versus surgery within multimodality treatment in stage III non-small cell lung cancer (NSCLC)- a cumulative meta-analysis of the randomized evidence. Oncotarget 2017;8:41670-8. [Crossref] [PubMed]
- Eberhardt WE, Poettgen C, Gauler T, et al. Phase III Study of Surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB Non-Small Cell Lung Cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- William WN, Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathological response and prediction of survival in patients with resectable non-small cell lung cancer after neo-adjuvant chemotherapy. J Thorac Oncol 2013;8:222-8. [Crossref] [PubMed]
- Agrawal V, Coroller T, Hou Y, et al. Radiologic-pathologic correlation of response to chemoradiation in resectable locally advanced NSCLC. Lung Cancer 2016;102:1-8. [Crossref] [PubMed]
- Pöttgen C, Levegrün S, Theegarten D, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res 2006;12:97-106. [Crossref] [PubMed]
- Cerfolio RJ, Bryant A, Winokur T, et al. Repeat FDG-PET after neo-adjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg 2004;78:1903-9. [Crossref] [PubMed]
- Kappers I, van Sandick J, Burgers J, et al. Results of combined modality treatment in patients with non-small cell lung cancer of the superior sulcus and the rationale for surgical resection. Eur J Cardiothorac Surg 2009;36:741-6. [Crossref] [PubMed]
- Arnett AL, Packard AT, Mara K, et al. FDG-PET parameters as predictors of pathologic response and nodal clearance in patients with stage III non-small cell lung cancer receiving neoadjuvant chemoradiation and surgery. Pract Radiat Oncol 2017;7:e531-e541. [Crossref] [PubMed]
- Fukumoto K, Fukui T, Okasaka T, et al. The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography for predicting pathologic response after induction therapy for thymic epithelial tumors. World J Surg 2017;41:1828-33. [Crossref] [PubMed]
- Kittaka H, Takahashi H, Ohigashi H, et al. Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg 2013;37:169-78. [Crossref] [PubMed]
- Cho YB, Chun H, Kim M, et al. Accuracy of MRI and 18F-FDG PET/CT for restaging after preoperative concurrent chemoradiotherapy for rectal cancer. World J Surg 2009;33:2688-94. [Crossref] [PubMed]
- de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer. Prediction of pathologic stage. J Thorac Oncol 2010;5:389-98. [Crossref] [PubMed]
- Pöttgen C, Gauler T, Bellendorf A, et al. Standardized uptake decrease on (18F)- fluorodeoxyglucose positron imission tomography after neo-adjuvant chemotherapy is a prognostic classifier for long-term outcome after multimodality treatment: secondary analysis of a randomized trial for resectable stage IIIA/B Non-Small Cell Lung Cancer. J Clin Oncol 2016;34:2526-33. [Crossref] [PubMed]
- Crabtree TD. Is there any role for positron emission tomography-computed tomography after induction therapy in locally advanced non-small cell lung cancer? J Thorac Cardiovasc Surg 2016;151:911-2. [Crossref] [PubMed]
- Coroller TP, Agrawal V, Narayan V, et al. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother Oncol 2016;119:480-6. [Crossref] [PubMed]


