Radiation dose effect in locally advanced non-small cell lung cancer
Introduction
Radiotherapy (RT) is needed in over 60% of patients with lung cancer at least once during the course of disease, adequate dose is an essential element for successful treatment of patients with non-small cell lung cancer (NSCLC). This article will briefly review biological considerations of radiation dose and their effect in the context of three-dimensional conformal radiation therapy (3D-CRT) including intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) for NSCLC. It will focus on literature review and discussions regarding radiation dose effect in locally advanced NSCLC including potential severe and lethal toxicities of high dose radiation given with concurrent chemotherapy. Potential approaches for delivering safe and effective doses by individualizing treatment are being applied in studies such as RTOG1106. The concept of delivering high dose radiation to the most resistant tumors with the use of isotoxic dose prescription and adaptive approaches will also be discussed in this paper.
Radiation dose effect: biology consideration
In the laboratory, from a biological effectiveness perspective, efficacy of radiation cell killing is directly correlated with the dose delivered. According to the basic principle of the linear-quadratic model, lethal radiation damage is created in one of two ways: as a consequence of a single ionizing event of double-strand breaks in the DNA or as a consequence of two, separate, sub-lethal ionizing events which interact pairwise to create lethal damage. As a result, the biological effect (E) of RT depends on the dose in a linear and quadratic fashion: E = n(αd+βd2) with n being the number of fractions, d being the dose per fraction, and α and β being parameters that determine the initial slope and curvature of the underlying cell-survival curve. From this equation, the biological effect dose (BED) can be calculated as: BED = nd [1+d/(α/β)] (1). BED varies according to dose per fraction, number of fractions and characteristics of the tissue contributing to the α/β ratio. BED is used to estimate the effect or risk of radiation in current practice of radiation oncology. When effects of equivalent total doses with different fractionation schemes are compared, they produce unequal biological effects (1). In lung cancer, early evidence suggests that the tumor control rate increases with escalation of BED (Figure 1) (2).
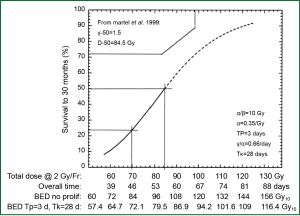
RT dose effect in NSCLC treated with conventionally fractionated 3D-CRT
While traditional radiation was previously more limited by technology for normal tissue sparing, modern 3D-CRT is able to deliver high-dose radiation to the tumor target areas while minimizing dose to surrounding tissues, allowing greater RT dose for early stage inoperable NSCLC patients (3-7). Dose has been escalated to up to 102.9 Gy while limiting lung dosimetry with most patients tolerating treatment, and post treatment radiation injuries considered to be acceptable (8). Increasing the dose of radiation improves local control and overall survival in most studies reported. In RTOG protocol 73-01 (9) it was found that the in-field failure rate decreased from 58% to 35% as the dose was increased from 40 to 60 Gy. In a phase I dose-escalation study reported by Rosenzweig et al. (10) the 2-year overall survival (OS) rate for patients with stage I-II disease who received <80 Gy was 60%, compared with 66% for patients who received >80 Gy (P<0.05), with a median survival time of 25.0 months versus 53.6 months, respectively. A prospective study reported by Kong et al. (3) found that the 5-year local-regional progression-free survival (PFS) rates were 12%, 35%, and 49% for groups treated with 67, 80, and 97 Gy, respectively. Median survival (5-year OS) in this study was 12 months (4%), 27 months (22%), and 22 months (28%) for dose levels of 63-69 Gy (mean =67 Gy), 74-84 Gy (mean =80 Gy) and 92-102 Gy (mean =97 Gy), respectively (P<0.0002) (Figure 2) (8). The dose response curve for local tumor control was steeper for five years than that of three or four years. Kong et al. from University of Michigan (8) demonstrated that high-dose radiation is more vital for patients with larger tumors and may be effective in reducing the adverse outcome associated with a large GTV in early stage NSCLC treated with conventionally fractionated radiation.
RT dose effect in early stage NSCLC treated with hypo-fractionated SBRT
A promising new technique, SBRT normally delivers much higher BED than conventionally fractionated 3D-CRT (typically BED of 70-85 Gy), and has generated outstanding tumor control in early stage NSCLC. High BED often contributes to long survival and good local tumor control. Studies from Japan, Germany and China all reported that SBRT with BED ≥100 Gy was associated with significantly better local control and long-term survival. In patients who received a BED ≥100 Gy, local tumor control was over 90%. A multicenter study (11) reviewed 257 patients treated at 14 institutions in Japan using a number of different treatment doses and delivery approaches. At median follow up of 38 months, local recurrence rate was 8.4% in patients who were treated to a BED ≥100 Gy. A recent German study also reported that BED ≥100 Gy is critical for achieving good local control (12). A Chinese study applied daily fractionated SBRT with a total BED of up to 115 Gy and reported 3- and 5-year OS rates for T1-3 patients of 57.3% and 35.1%, respectively, and 60.2 and 36.5% 3- and 5-year OS rates for stage T1-2 patients respectively (13). Studies from the U.S. suggest that patients who receive 16 Gy ×3 (BED =124 Gy) have significantly better local control than those who receive lower doses (14). Dose response analysis showed that the outcome plateaued around 120 Gy BED. In Guckenberger’s study (12), a PTV-encompassing dose of ≥100 Gy BED was estimated to be required for local tumor control rates >90%. RTOG 0236 (15), using 18 Gy ×3, equating to a BED of 180 Gy to tumor, represented the First National Cancer Institute cooperative group trial using SBRT for early NSCLC. The study reported 98% tumor control rate at three years. Updated Japanese (16) and German (17) studies of BED above 100 Gy confirmed over 90% local tumor control for T1 tumors. However, there is no randomized trial to compare different dose regimens for SBRT. In a meta-analysis containing 34 published SBRT datasets (18), observed 5-year OS and cancer specific survival (CSS) was best in those treated to medium BED (around 100 Gy).
Modern technology also allows SBRT delivery of very high radiation dose to the target volume, in as few as one single fraction. However, the effects of radiation after SBRT in a single fraction are not well known. In lung metastases patients receiving a dose of 30 Gy in a single fraction therapy It was reported that LC rates at one and two years were 89.1% and 82.1%, OS rates were 76.4% and 31.2%, CCS rates were 78.5% and 35.4%, and PFS rates were 53.9% and 22%, respectively (19). Interestingly, Guckenberger et al. (20) reported that the dose-response relationship was limited in fractionated SBRT: LC was independent from the irradiation dose in the subgroup of patients treated with single-fraction SBRT. Nevertheless, adequate radiation dose is important for good tumor control and survival in early stage NSCLC and the success of hypofractionated high dose SBRT is a strong testimony for radiation dose effect in patients treated with hypofractionated techniques (3 to 8 fractions).
RT dose effect in locally advanced NSCLC treated with chemoradiation
In locally advanced NSCLC, there are two important aspects to consider: (I) does local regional tumor control impact survival in patients with locally advanced disease, with high risk of distant disease spread? (II) with extensive tumor involvement in the chest which hosts critical structures, would high dose radiation cause significant toxicity adversely impacting patients? Ultimately, it is important to address whether high dose radiation improves overall survival and quality of life.
Local-regional tumor control and overall survival in locally advanced NSCLC
Local tumor progression is common, and remains a major problem after radiation-based non-surgical treatment in locally advanced NSCLC, despite of advances in radiation technology. Using modern techniques, current radiation therapy applying a uniform dose prescription of 60 Gy or slightly higher generates local control rates of less than 50% and a 5-year overall survival rate of about 10-15% (8,21,22). After RT with or without neoadjuvant chemotherapy, Kong et al. in a University of Michigan trial reported ultimate local failure in 70% of patients (8). After neoadjuvant chemoradiotherapy in CALGB 9431 (23), 90% of patients ultimately failed locally, with 45% having local failure alone. After neoadjuvant and concurrent chemotherapy with radiation doses of 60-74 Gy, Socinski et al. (24) reported that 46% of patients initially had local failure. Evaluation by bronchoscopy and biopsy one year after treatment completion revealed pathologic local control rates of only 15-17% after 65 Gy of radiation with neoadjuvant therapy (25). After chemoradiation with RT doses of 60 Gy in 2 Gy daily fractions or 69.6 Gy in 1.2 Gy twice daily fractions, a secondary analysis of 11 RTOG trials (9/11 had concurrent chemoradiation) with 1,356 patients reported 2- and 5-year survival rates of 38% and 15%, with 2- and 5-year local-regional failure (LRF) rates of 46% and 52%, respectively (26).
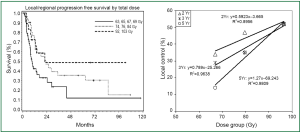
Local-regional disease not only leads to death due to local effects within the chest, but also can serve as a source for metastatic dissemination. In patients with locally advanced disease, Arriagada (27) concluded that the main cause of failure is the absence of local control, and local progression or relapse correlated with poorer survival. In RTOG 73-01 (9), the death rate in patients with intra-thoracic failure was similar to that of patients with distant metastases, and increased survival was observed in patients with complete tumor response (28). In the CHART trial, local control rates of 20% and 29% were associated with median survivals of 9.9 and 27.9 months, respectively (29). In an EORTC trial, Schaake-Koning et al. (30) demonstrated a similar correlation between LRC and survival. Reviewing mature results of ten randomized phase III trials with inclusion of concurrent chemoradiation, Auperin et al. (31) reported local or local regional control along with overall survival; there seemed significant correlation between LRC and survival rates (Figure 3) (32-37).
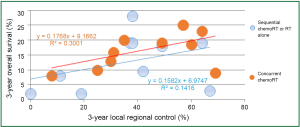
RT dose, fraction and survival in locally advanced NSCLC
In locally advanced NSCLC, 5-year OS rate is only about 15% after conventionally fractionated 60 Gy radiation. Dose escalation trials using involved field radiation therapy have demonstrated improved outcomes for patients treated to higher radiation doses, however only a few studies have investigated efficacy and tolerance. The Memorial Sloan Kettering Cancer Center (MSKCC) conducted a phase I dose escalation study of stage IIIA/B patients who received radiation dose of 70.2 to 84 Gy in 1.8 Gy fractions; the OS was significantly superior in patients who received ≥80 Gy (38). In a randomized trial from China, 5-year LC and 2-year OS improved significantly in stage III patients treated with total dose of 68-74 Gy compared with those treated to 60-64 Gy (51% vs. 36%, P=0.032; 39.4% vs. 25.6%, P=0.048) (39). Hypo-fractionated RT regimens can also increase the dose to the tumor volume based on the concept that a higher dose per fraction can increase BED, though there are no randomized trials comparing benefits and tolerance among Hypo-fractionated RT and standard schedules. A study by Zhu et al. (40) performed dose escalation up to 65-68 Gy in 22 to 23 fractions in 34 NSCLC patients with stage III at diagnosis. 2-year OS, PFS, and LPFS rates were 38%, 30%, and 61%, respectively. In a recent study (41) reported by Osti et al., 24 stage IIIA/B patients had a median OS of 13 months (16 months for IIIA; 13 months for IIIB), with a range of 4 to 56 months. BED >55 Gy was significantly associated with survival benefit (P<0.001). Another hypo-fractionated RT study (42) included 37 stage III patients without administration of concurrent chemotherapy. All patients were treated with 25 fractions, with dose per fraction ranging from 2.28 to 3.22 Gy. The outcome data showed that 17% of patients achieved complete response, the actuarial 2-year OS calculated to be 46.8%±9.7%, with median survival of 18 months. Hyper-fractionated accelerated RT is another method to elevate BED to the tumor. In order to increase total dose to tumor while shortening treatment duration and decreasing late effects, hyper-fractionated-accelerated RT has been attempted in IIIA/B NSCLC patients. In 127 patients receiving hyper-fractionated-accelerated RT, Jeremić et al. (43) reported 5-year OS, local PFS and distant metastasis-free survival of 7%, 16%, and 36%, respectively. After two cycles of chemotherapy, stage III NSCLC patients in the DART-bid trial (44) had median OS of 24.3 months, and 2-/5-year OS rates to 51% and 18%, respectively. In a randomized phase III trial reported by Baumann et al. (45), survival after conventional RT and Hyper-fractionated-accelerated RT was not different, while local control after Hyper-fractionated-accelerated RT was significantly better than control after conventional RT in patients who had received chemotherapy before RT (P=0.019).
RT dose effect in locally advanced NSCLC treated with concurrent chemoradiation
In the standard care for locally advanced NSCLC: platinum based chemotherapy concurrent with RT, local tumor control and overall survival remain poor. After neo-adjuvant and concurrent chemotherapy with radiation doses of 60-74 Gy, Socinski et al. (46) reported that 46% of patients initially had local failure. A secondary analysis of 11 RTOG trials (9/11 had concurrent chemoradiation) with 1,356 patients treated with chemoradiation with RT doses of 60 Gy in 2 Gy daily fractions or 69.6 Gy in 1.2 Gy twice daily fractions reported 2- and 5-year OS rates of 38% and 15%, with 2- and 5-year LRF rates of 46% and 52%, respectively (25). With concurrent chemotherapy, RTOG 92-04 reported that 2- and 4-year in-field progression (TTPs) were 26% and 30% in the patients receiving radiation dose of 69.6 Gy, compared to 45% and 49% in the 63 Gy arms (47).
RT dose may be an important factor for local tumor control and perhaps survival in this patient population. A good example is a report of 237 patients with stage III NSCLC treated with radiation +/– chemotherapy between 1992 and 2002 at the University of Michigan which showed that BED was the most significant prognostic factor associated with the risk of death (HR =0.96 for each Gy, 95% CI: 0.95-0.97, P<0.001). For patients who received concurrent chemotherapy, the hazard ratio of BED for the risk of death was 0.97 per Gy (95% CI: 0.95-0.99, P=0.013). One Gy of dose escalation was associated with a 3% reduction in the risk of death. BED remained a significant independent prognostic factor in patients treated with chemoradiation in the dose range of 60-66 Gy (HR =0.91, 95% CI: 0.84-0.99, P=0.041) (48). The RTOG secondary analysis of 1,356 patients treated with chemoradiation between1988 to 2002 serves as a good example of this as well. This study analyzed for BED effect (1,348 for treatment time adjusted BED~tBED) in the range of 60 Gy in 2 Gy fractions and 69.6 Gy in 1.2 Gy fractions. The 2- and 5-year OS rates were 38% and 15%, respectively. The 2- and 5-year LRF rates were 46% and 52%, respectively. BED (and tBED) was significantly associated with both OS and LRF, with or without adjustment for other covariates on multivariate analysis (P<0.0001). A 1-Gy BED increase in RT dose intensity was significantly associated with approximately 4% relative improvement in survival (HR for death =0.96) and 3% relative improvement (HR =0.97) in local-regional control (26).
Overall, radiation dose escalation may improve local regional control and overall survival in patients with stage III NSCLC, based on the results of non-randomized trials (8,48-50) and an RTOG secondary analysis (26) of over 1,300 cases treated with chemoradiation. Regarding the dose effect of >70 Gy with concurrent chemoradiation, investigators from University of Michigan reported results on patients treated in the dose range of 60-100 Gy with concurrent and adjuvant carboplatin and paclitaxel (51). The median local-regional PFS was 10.7 (range: 8.4-13.0) months and has not yet been reached (14.1 to date) (P=0.001) for physical doses <70 and >70 Gy, respectively. The median survival was 15.5 (range: 6.5-24.4) months and 41.9 (range: 18.3-65.5) months (P=0.003), for physical doses less than and greater than 70 Gy, respectively. The RT dose effect was statistically significant for patients treated with or without concurrent chemotherapy (Figure 4).
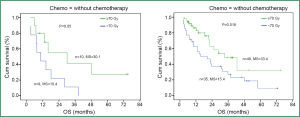
Challenges in delivering high dose radiation in locally advanced NSCLC
Treatment effect and toxicity after dose escalated RT
It is a remarkable challenge to deliver high dose radiation in patients with advanced NSCLC. A dose escalation study of 79 patients with locally advanced NSCLC treated without chemothereapy reported a maximum tolerance dose og 63.25 Gy in 25 daily fractions over five weeks using intensity-modulated RT to limit severe toxicity to 20%. Grade 4 to 5 late toxicities were attributable to damage to central and perihilar structures and correlated with dose to the proximal bronchial tree (52-54). A trial from University of Michigan with concurrent carboplatin and paclitaxel (UMCC 2003-073) was stopped prematurely due to lack of dose escalation in 60% of patients limited by clinical lung toxicity at 15%. RTOG 0117, a phase I/II dose escalation study with concurrent and adjuvant carboplatin and paclitaxel, reported two acute, treatment-related dose limiting toxicities (DLTs) in the 1st cohort of 17 patients and 6/8 (75%) grade ≥3 events during long-term follow up. The protocol was revised to de-escalate the radiation therapy dose (74 Gy in 37 fractions). In the new cohort of seven patients, treated with 74 Gy, there was 1 DLT in the first five patients and no DLTs in the next two patients. The maximum tolerable dose was thus determined to be 74 Gy in 37 fractions (2 Gy per fraction) using 3D-CRT with concurrent paclitaxel and carboplatin therapy (55). The CALBG 30105 trial (11) studied induction chemotherapy followed by concurrent chemoradiotherapy in stage III NSCLC patients randomised between two different chemotherapy regimens delivered concurrently with dose-escalated thoracic conformal RT (74 Gy, once daily, 2 Gy per fraction) in both arms. The carboplatin/gemcitabine arm closed prematurely due to a high rate of grade 4 to 5 pulmonary toxicity. However the carboplatin/paclitaxel arm demonstrated a median survival of 24 months with a 12% rate of grade 3 or higher pulmonary toxicity.
These trial results compared favorably to the historical standard concurrent chemoradiotherapy doses of 60-66 Gy in 2 Gy fractions.and formed the basis for the experimental arm in the recently closed phase III RTOG 0617 trial. In this 2×2 factorial design trial patients with stage III NSCLC were treated with weekly carboplatin-paclitaxel chemotherapy and concurrent RT in 2 Gy fractions. Patients were randomised to receive 60 or 74 Gy RT, with or without cetuximab. After RT, all patients received a further two cycles of consolidation chemotherapy, with or without cetuximab. A planned interim analysis after 85 documented events demonstrated a non-superior median survival in the high dose arms which were closed due to a low likelihood of survival benefit from high dose RT with additional accrual and follow up. An updated analysis of the data after 207 events demonstrated a significant increased risk of death in the high dose arms [median survival 28.7 (60 Gy arm) vs. 19.5 months (74 Gy), P=0.0007; HR =1.56, 95% CI: 1.19-2.06], with a 37% increased risk of local failure in the high dose arms (HR =1.37, 95% CI: 0.99 to 1.89, P=0.0319). There were more treatment related deaths in the high dose arms (10 vs. 2) but this did not reach statistical significance. The worse local control and survival of the high dose arms of RTOG 0617 trial has challenged the assumption that RT dose escalation using conventional dose/fractionation regimens with concurrent chemotherapy will improve outcome in stage III NSCLC. At the time of writing this article, the reasons for the underperformance of the 74 Gy arm are still unclear and the analysis of the individual RT plans by RTOG is ongoing. Hypotheses for the worse local control in the 74 Gy arms include issues with the assessment of local progression versus fibrosis, chemotherapy and RT dose delivery and compliance, issues with RT planning and quality assurance (particularly since IMRT was only used in 46% of centers) and accelerated repopulation due to the prolongation of the overall treatment time. This is supported by an early analysis estimating that tumor control probability of NSCLC decreases 1.6% per day after a six-week duration of RT, and according to a secondary analysis of three RTOG trials for stage III NSCLC patients treated with concurrent chemoradiotherapy, showing that prolonged treatment time translated into a 2% increase in the risk of death for each day of prolongation in therapy (56). A combination of factors probably account for the survival results of RTOG 0617, including inferior local control in the 74 Gy arms; but unreported treatment-related deaths (cardiac and pulmonary) are likely to be one of the major causes for the inferior survival in the 74 Gy arms. Indeed the multivariate survival analysis reported that V5 and V50 heart were both associated with worse survival. This study highlights the need for stricter constraints to adjacent critical organs at risk such as heart, lung, proximal bronchial tree and RT quality assurance programs in future studies and institutional protocols. The current view in the radiation oncology community is that radiation dose escalation with conventional fractionation and concurrent CT is not the way forward, but treatment intensification should be pursued, including studies of altered fractionation and individualization of dose (57-59).
Currently, there are investigative efforts to increase daily fraction size to escalate total radiation dose without extending the treatment duration. One approach involves dose escalation using 2.25 Gy daily fractions (once or twice daily) while limiting treatment duration to six weeks (60). This approach was used to escalate to 87.8 Gy in patients with limited lung volumes without concurrent chemotherapy. Another approach is to use a higher dose fraction every day while limiting the treatment duration to five weeks without concurrent chemotherapy (61). UMCC 200373 and UMCC2007123 limited treating duration to six weeks while delivering RT dose escalation with concurrent chemotherapy, and achieved promising results (51).
Treatment related death after RT based treatment
Treatment related severe toxicities can be fatal. For example, a recent meta-analysis reported 1.9% grade 5 pneumonitis after concurrent chemoradiotherapy (62). Radiation pneumonitis attributed death occurred in up to 10% (35,63,64) of patients treated with concurrent chemoradiation, and up to 4.3% of patients treated with radiation alone (35,65,66). Critical organs at risk include the heart, lung and esophagus. Grade 5 adverse events were reported in 1.7% (range, 1-3%) (67,68), and 2.5% (range, 1.2-8.2%) (69,70), for patients treated with concurrent chemotherapy with conventional doses (60-63 Gy) and concurrent chemotherapy with escalated doses (>63 Gy). It is possible that these increased events were due to treatment toxicity, though some of them were not identified as such. Another ongoing issue with the reporting of treatment related deaths is that many patients die at home or at local community hospitals, leading to probable underreporting of grade 5 events. These treatment toxicities often arise as a consequence of the challenges of delivering high dose radiation to locally advanced disease without incidentally delivering high dose to the OARs (Table 1).
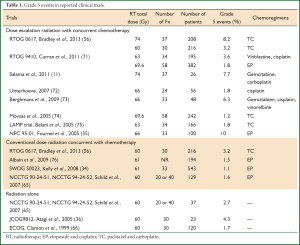
Full table
Potential strategies to improve therapeutic gain in NSCLC
It is imperative to pursue new strategies to increase the dose ratio of tumor target over critical structures. Radiation physics and technology advancements such as IMRT, IGRT, and volume based planning are important for delivery of radiation precisely to the target, though this will not be discussed in this review. Knowledge of tumor target gained from tools such as Positron Emission Tomography helps define the target more accurately. Individualized radiation with isotoxicity prescription is a promising strategy. For traditional adaptive radiation plan, prescription dose is required to cover the whole GTV and CTV determined according to images simulated before therapy. To obtain the best LRC and OS from radiation, higher total dose while limiting total treatment duration less than six weeks and dosimetric factors such as V20 and MLD should be seriously considered especially for larger tumors (diameter >5 cm). An ongoing European phase II PET-boost trial (ClinicalTrials.gov Identifier: NCT01024829) randomises patients with stage IB-III NSCLC to dose-escalation starting from 66 Gy given in 24 fractions of 2.75 Gy with an integrated boost to either the entire primary tumour or to >50% of the maximum Standardised Uptake Volume (SUVmax) area of the primary tumor, while limiting MLD to 20 Gy. Preliminary results from the first 20 randomised patients showed that this was feasible and did not exceed pre-defined normal tissue constraints. Recent studies from Kong et al. at University of Michigan (3) demonstrated that there is a significant decrease in tumor size and FDG activity after radiation dose of 45 Gy. According to this result, we could adapt targeting to the decreased tumor defined on FDG-PET/CT after 45 Gy with a fixed composite MLD limit of 20 Gy while allowing remarkable escalation of total dose to the tumor. Kong et al. have demonstrated that tumor volume reduces significantly more on FDG PET than on CT at 40-50 Gy (4-5 weeks during the course of fractionated RT) (77). Using the reduced volume identified on during-RT PET, dose to active and resistent tumor was significantly escalated while dose to the normal tissues were either reduced (due to adaptive shrinking fields) or unchanged (78). The ongoing RTOG1106 trial (http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1106) adopted this concept, and will use this approach to obtain FDG-PET/CT during the course of chemoradiation to adapt their plan to a tumor target smaller than that from before therapy to escalate dose to as high as 80.4 Gy delivered in six weeks without increasing doses to the OARs. The total dose for each patient in the experimental arm will be determined by the dose corresponding to a MLD of 20 Gy (equivalent to a 15-17% probability of grade >2 lung toxicity based on the current NTCP model). The study hypothesized that the during-treatment PET/CT-based adaptive therapy will allow us to dose escalate (i.e., raise the daily dose to the reduced target volume for the remainder of the treatment) in the majority of patients and meet the dose limits of normal structures, thus improving LRC without increasing normal tissue toxicity. This will also allow us to use the lung dose limits to individualize adaptive dose escalation to residual active tumor regions and limit the incidence of pneumonitis and other toxicities simultaneously.
Conclusions
In summary, there is a clear radiation dose effect in NSCLC patients. Although the benefit of high dose radiation has been demonstrated in early stage patients, the clinical benefit of high dose radiation in patients has been challenged by preliminary results from RTOG0617. Treatment related toxicity can be a major reason for failure of high dose radiation. Future study of radiation therapy may benefit from individualized radiation dose prescription based on the sensitivity of tumor and critical organs of each individual patient. Studies from Europe will individualize doses based on FDG intensity at baseline while limiting treatment duration to five weeks. RTOG1106, an ongoing randomized phase II study, will examine the effect of individualized adaptive radiation therapy (over an uniform 60 Gy) by targeting high dose radiation to most resistant tumor while keeping doses to critical structures strictly controlled in locally advanced NSCLC patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- O’Rourke SF, McAneney H, Hillen T. Linear quadratic and tumour control probability modelling in external beam radiotherapy. J Math Biol 2009;58:799-817. [PubMed]
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999;24:31-7. [PubMed]
- Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys 2006;65:1075-86. [PubMed]
- Socinski MA, Marks LB, Garst J, et al. Carboplatin/paclitaxel or carboplatin/vinorelbine followed by accelerated hyperfractionated conformal radiation therapy: a preliminary report of a phase I dose escalation trial from the Carolina Conformal Therapy Consortium. Oncologist 2001;6 Suppl 1:20-4. [PubMed]
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005;61:318-28. [PubMed]
- Song Y, Mueller B, Obcemea C, et al. Feasibility of implementing stereotactic body radiation therapy using a non-commercial volumetric modulated arc therapy treatment planning system for early stage lung cancer. Conf Proc IEEE Eng Med Biol Soc 2011;2011:409-12.
- Sonke JJ, Rossi M, Wolthaus J, et al. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. Int J Radiat Oncol Biol Phys 2009;74:567-74. [PubMed]
- Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324-33. [PubMed]
- Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874-81. [PubMed]
- Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer 2005;103:2118-27. [PubMed]
- Salama JK, Stinchcombe TE, Gu L, et al. Pulmonary toxicity in Stage III non-small cell lung cancer patients treated with high-dose (74 Gy) 3-dimensional conformal thoracic radiotherapy and concurrent chemotherapy following induction chemotherapy: a secondary analysis of Cancer and Leukemia Group B (CALGB) trial 30105. Int J Radiat Oncol Biol Phys 2011;81:e269-74. [PubMed]
- Guckenberger M, Wulf J, Mueller G, et al. Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys 2009;74:47-54. [PubMed]
- Chen Y, Guo W, Lu Y, et al. Dose-individualized stereotactic body radiotherapy for T1-3N0 non-small cell lung cancer: long-term results and efficacy of adjuvant chemotherapy. Radiother Oncol 2008;88:351-8. [PubMed]
- Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol 2007;30:637-44. [PubMed]
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [PubMed]
- Wulf J, Baier K, Mueller G, et al. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 2005;77:83-7. [PubMed]
- Zhang J, Yang F, Li B, et al. Which Is the Optimal Biologically Effective Dose of Stereotactic Body Radiotherapy for Stage I Non-Small-Cell Lung Cancer? A Meta-Analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [PubMed]
- Osti MF, Carnevale A, Valeriani M, et al. Clinical outcomes of single dose stereotactic radiotherapy for lung metastases. Clin Lung Cancer 2013;14:699-703. [PubMed]
- Guckenberger M, Klement RJ, Allgauer M, et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiother Oncol 2013;109:13-20. [PubMed]
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210-5. [PubMed]
- Sause W, Kolesar P, Taylor S IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358-64. [PubMed]
- Vokes EE, Herndon JE 2nd, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol 2002;20:4191-8. [PubMed]
- Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: a modified phase I/II trial. Cancer 2001;92:1213-23. [PubMed]
- Tursz T, Cesne AL, Baldeyrou P, et al. Phase I study of a recombinant adenovirus-mediated gene transfer in lung cancer patients. J Natl Cancer Inst 1996;88:1857-63. [PubMed]
- Machtay M, Bae K, Movsas B, et al. Higher Biologically Effective Dose of Radiotherapy Is Associated with Improved Outcomes for Locally Advanced Non-Small Cell Lung Carcinoma Treated with Chemoradiation: An Analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2012;82:425-34. [PubMed]
- Arriagada R. Current strategies for radiation therapy in non-small cell lung cancer. Chest 1997;112:209S-213S. [PubMed]
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 1992;326:524-30. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999;52:137-48. [PubMed]
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Radiosensitization by cytotoxic drugs. The EORTC experience by the Radiotherapy and Lung Cancer Cooperative Groups. Lung Cancer 1994;10 Suppl 1:S263-70. [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [PubMed]
- Lu C, Lee JJ, Komaki R, et al. Chemoradiotherapy with or without AE-941 in stage III non-small cell lung cancer: a randomized phase III trial. J Natl Cancer Inst 2010;102:859-65. [PubMed]
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008;26:5755-60. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 2005;23:5910-7. [PubMed]
- Atagi S, Kawahara M, Tamura T, et al. Standard thoracic radiotherapy with or without concurrent daily low-dose carboplatin in elderly patients with locally advanced non-small cell lung cancer: a phase III trial of the Japan Clinical Oncology Group (JCOG9812). Jpn J Clin Oncol 2005;35:195-201. [PubMed]
- Belani CP, Wang W, Johnson DH, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol 2005;23:3760-7. [PubMed]
- Le QT, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol 2006;1:802-9. [PubMed]
- Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol 2007;30:239-44. [PubMed]
- Zhu ZF, Fan M, Wu KL, et al. A phase II trial of accelerated hypofractionated three-dimensional conformal radiation therapy in locally advanced non-small cell lung cancer. Radiother Oncol 2011;98:304-8. [PubMed]
- Osti MF, Agolli L, Valeriani M, et al. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:e157-63. [PubMed]
- Adkison JB, Khuntia D, Bentzen SM, et al. Dose escalated, hypofractionated radiotherapy using helical tomotherapy for inoperable non-small cell lung cancer: preliminary results of a risk-stratified phase I dose escalation study. Technol Cancer Res Treat 2008;7:441-7. [PubMed]
- Jeremić B, Miličić B, Milisavljevic S. Concurrent hyperfractionated radiation therapy and chemotherapy in locally advanced (Stage III) non-small-cell lung cancer: single institution experience with 600 patients. Int J Radiat Oncol Biol Phys 2012;82:1157-63. [PubMed]
- Wurstbauer K, Deutschmann H, Dagn K, et al. DART-bid (Dose-differentiated accelerated radiation therapy, 1.8 Gy twice daily)--a novel approach for non-resected NSCLC: final results of a prospective study, correlating radiation dose to tumor volume. Radiat Oncol 2013;8:49. [PubMed]
- Baumann M, Herrmann T, Koch R, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol 2011;100:76-85. [PubMed]
- Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol 2004;22:4341-50. [PubMed]
- Komaki R, Seiferheld W, Ettinger D, et al. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non-small-cell lung cancer: long-term follow-up of RTOG 92-04. Int J Radiat Oncol Biol Phys 2002;53:548-57. [PubMed]
- Wang L, Correa CR, Zhao L, et al. The effect of radiation dose and chemotherapy on overall survival in 237 patients with Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;73:1383-90. [PubMed]
- Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2002;52:49-57. [PubMed]
- Rengan R, Rosenzweig KE, Venkatraman E, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;60:741-7. [PubMed]
- Kong FM, Ten Haken R, Hayman J, et al. Personalized High Dose Radiation (> 70 Gy) Is Significantly Associated with Better Local Regional Control and Overall Survival in Non-small Cell Lung Cancer Treated with Concurrent Chemoradiation. Int J Radiat Oncol Biol Phys 2011;81:S594.
- Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 2013;31:4343-8. [PubMed]
- Han C, Wang W, Quint L, et al. Relationship Between Pulmonary Artery Invasion and High-dose Radiation and Overall Survival in Patients With Non-small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2012;84:S612.
- Xue J, Han C, Matuszak M, et al. High Dose to Large Volumes of Pericardium May Be Associated With Radiation-related Pericardial Effusion and Survival in Patients With NSCLC. Int J Radiat Oncol Biol Phys 2012;84:S592-3.
- Bradley JD, Moughan J, Graham MV, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 2010;77:367-72. [PubMed]
- Bradley JD, Komaki R, Masters GA, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. J Clin Oncol 2013;31:abstr 7501.
- Ben-Dor I, Waksman R, Hanna NN, et al. Utility of radiologic review for noncardiac findings on multislice computed tomography in patients with severe aortic stenosis evaluated for transcatheter aortic valve implantation. Am J Cardiol 2010;105:1461-4. [PubMed]
- Zhao KL, Ma JB, Liu G, et al. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys 2010;76:446-51. [PubMed]
- Cheng Y, Anick P, Hong P, et al. Temporal relation discovery between events and temporal expressions identified in clinical narrative. J Biomed Inform 2013;46 Suppl:S48-53. [PubMed]
- Belderbos JS, De Jaeger K, Heemsbergen WD, et al. First results of a phase I/II dose escalation trial in non-small cell lung cancer using three-dimensional conformal radiotherapy. Radiother Oncol 2003;66:119-26. [PubMed]
- Mehta M, Scrimger R, Mackie R, et al. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001;49:23-33. [PubMed]
- Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50. [PubMed]
- Bral S, Duchateau M, Versmessen H, et al. Toxicity report of a phase 1/2 dose-escalation study in patients with inoperable, locally advanced nonsmall cell lung cancer with helical tomotherapy and concurrent chemotherapy. Cancer 2010;116:241-50. [PubMed]
- Naito Y, Kubota K, Nihei K, et al. Concurrent chemoradiotherapy with cisplatin and vinorelbine for stage III non-small cell lung cancer. J Thorac Oncol 2008;3:617-22. [PubMed]
- Schild SE, Mandrekar SJ, Jatoi A, et al. The value of combined-modality therapy in elderly patients with stage III nonsmall cell lung cancer. Cancer 2007;110:363-8. [PubMed]
- Clamon G, Herndon J, Cooper R, et al. Radiosensitization with carboplatin for patients with unresectable stage III non-small-cell lung cancer: a phase III trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol 1999;17:4-11. [PubMed]
- Saitoh J, Saito Y, Kazumoto T, et al. Concurrent chemoradiotherapy followed by consolidation chemotherapy with bi-weekly docetaxel and carboplatin for stage III unresectable, non-small-cell lung cancer: clinical application of a protocol used in a previous phase II study. Int J Radiat Oncol Biol Phys 2012;82:1791-6. [PubMed]
- Phernambucq EC, Hartemink KJ, Smit EF, et al. Tumor cavitation in patients with stage III non-small-cell lung cancer undergoing concurrent chemoradiotherapy: incidence and outcomes. J Thorac Oncol 2012;7:1271-5. [PubMed]
- Byhardt RW, Vaickus L, Witt PL, et al. Recombinant human interferon-beta (rHuIFN-beta) and radiation therapy for inoperable non-small cell lung cancer. J Interferon Cytokine Res 1996;16:891-902. [PubMed]
- Graham PH, Clark C, Abell F, et al. Concurrent end-phase boost high-dose radiation therapy for non-small-cell lung cancer with or without cisplatin chemotherapy. Australas Radiol 2006;50:342-8. [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Uitterhoeve AL, Koolen MG, van Os RM, et al. Accelerated high-dose radiotherapy alone or combined with either concomitant or sequential chemotherapy; treatments of choice in patients with Non-Small Cell Lung Cancer. Radiat Oncol 2007;2:27. [PubMed]
- Berghmans T, Van Houtte P, Paesmans M, et al. A phase III randomised study comparing concomitant radiochemotherapy as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2009;64:187-93. [PubMed]
- Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol 2005;23:2145-54. [PubMed]
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005;23:5883-91. [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [PubMed]
- Kong FM, Frey KA, Quint LE, et al. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol 2007;25:3116-23. [PubMed]
- Mahasittiwat P, Yuan S, Xie C, et al. Metabolic Tumor Volume on PET Reduced More than Gross Tumor Volume on CT during Radiotherapy in Patients with Non-Small Cell Lung Cancer Treated with 3DCRT or SBRT. J Radiat Oncol 2013;2:191-202. [PubMed]


