Ultrasound-guided pleural cutting needle biopsy: accuracy and factors influencing diagnostic yield
Introduction
Pleural effusion (PE) is a common medical problem with more than 50 recognized causes (1). Approximately 3,000 new cases of PE per 1,000,000 of the population are recorded annually in industrialized countries (2). Most exudative PEs are malignant, tuberculous, or parapneumonic (3,4).
It is difficult to determine the cause of PE, particularly malignant PE, via thoracocentesis alone; the diagnostic accuracy is relatively low (2,5,6). Pleural biopsy (PB) followed by histological evaluation is important when diagnosing PE. Diagnostic tissue can be acquired using several procedures, such as blind PB, local anaesthetic thoracoscopy (LAT), VATS, or image-guided biopsy (4,7,8). Medical thoracoscopic, VATS, and image-guided pleural biopsies are more sensitive than blind PB (5,9,10). However, medical thoracoscopy (MT) and VATS require a degree of expertise, anesthesia, and the use of an operating theater (10,11). Image-guided biopsies lack these shortcomings and are clearly better than blind PB, particularly if malignant PE is suspected (9). The reported sensitivities range from 61% to 94%, and a few studies have reported 100% specificity (9,12-17).
The ultrasound and CT seem to exhibit similar diagnostic yields (18). However, ultrasound (US)-guided biopsy is particularly rapid and inexpensive and is associated with a low incidence of post-procedure pneumothorax (19). The method allows real-time visualization of the biopsy needle without exposing patients or doctors to radiation. Heavy or rapid breathing of dyspneic patients can be accommodated via real-time ultrasonic guidance. The literature indicates that the diagnostic accuracy of US-guided percutaneous needle biopsy (PCNB) is 62.9% to 94% (12-15). Because of its advantages, US is commonly used to guide biopsy in clinical practice. However, to the best of our knowledge, few reports have explored factors influencing the diagnostic yield of US-guided PCNB. Here we retrospectively analyze a large number of cases.
Methods
Patients
We conducted a retrospective study at Guangzhou Institute of Respiratory Disease. This study was approved by the Scientific Research Ethics Review Committee of the First Affiliated Hospital of Guangzhou Medical University. We retrospectively analyzed data from 147 percutaneous US-PCNBs performed on 144 patients between July 2014 and June 2016. The inclusion criteria were (I) undiagnosed and untreated PE, (II) a unilateral transudate evident on clinical images that did not resolve on treatment, and (III) age >18 years. The exclusion criteria were (I) insufficient bleeding diathesis to allow for pleural aspiration and biopsy, (II) patient inability to provide written informed consent, or (III) non-malignant and unclearly diagnosed cases were followed up for less than 12 months or lost to follow-up.
Procedure
All procedures were similar, with patients placed in either the sitting, prone, supine, or lateral decubitus position. First, US (Esaote Mylab 90, Italy) using a low-frequency (2–5 MHz) convex transducer was used to collect information on the PE, the pleura, and blood flow. To maximize accuracy, the thickest point of the pleura or a focally thickened region was chosen for biopsy on the premise of security. If such an area was not available, the entry points were selected to be as close to the diaphragm as possible. If patients had undergone prior chest computed tomography (CT), the CT scans were also examined. Next, both low-frequency (2–5 MHz) and high-frequency (5–10 MHz) probes were alternately used to guide the PB, which was performed by two operators. The biopsy plan was decided by the operators in consultation. Operator 1 was a sonographer who assessed the condition of the pleura and also provided the guidance. Operator 2 used an 18 or 16 G automated cutting needle with a specimen notch of 20 mm (MC1816, Bard Max. Core, Bard Inc., USA) to perform the biopsy with the patient under local anesthesia with 2% lidocaine. The tip of the cutting needle was inserted through the guide channel into the pleural superstratum. We found it wise to program a launch distance ≥22 mm to avoid lung damage. The number of punctures depended on the quality of the specimens and the patient’s tolerance. All specimens were immediately fixed in 10% formalin and sent for histopathological examination. All US procedures, including pleural ultrasonic examination and real-time guidance, were performed by two experienced interventional sonographers (DZ Zhou and XH Zhou). All biopsies were performed by these sonographers or an experienced pulmonologist (JL Wang).
Prior to PB, thoracocentesis was used to obtain PE samples from all patients for biochemical and microbiological analysis. The Abrams biopsy was performed in cases of moderate and large effusion after US-guided PB. We routinely used US to check whether a pneumothorax and/or active intrathoracic bleeding was present. If a pneumothorax or a related symptom was suggested, we scheduled further radiological examination.
Diagnostic assessment
Diagnoses were classified as malignant, specifically benign [e.g., benign mesothelioma, tuberculosis (TB) and eosinophilic infection], nonspecifically benign (e.g., nonspecific inflammation), or inconclusive. Malignancy and specifically benign disease were considered positive findings, and nonspecific benign disease was considered a negative finding. An inconclusive diagnosis indicated that the biopsy had not been completed because of complications and the pathological results were not definitive (e.g., granulomatous inflammation).
A definitively positive diagnosis, including malignancy or specifically benign disease, was made via histopathological analysis of the US-PCNB samples or if other sites revealed the same histological characteristics, metastasis was identified, followed by surgery or clinical treatment. A definitively negative diagnosis was made by histopathological analysis, if the PE subsequently disappeared, or if follow-up chest radiographs or CT scans showed that the PE remained stable for ≥12 months after corresponding treatment. True-positive and true-negative cases belong to correct diagnoses. False-positive, false-negative, and inconclusive cases belong to incorrect diagnoses.
Variables
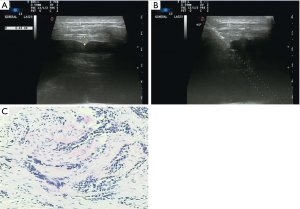
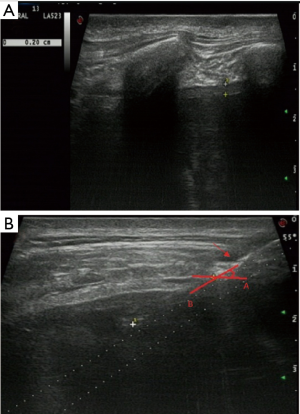
Variables were classified into three categories: patient, pleural, and biopsy-related factors. Patient factors included patient age and sex. Pleural factors included pleural thickness and morphology. Pleural thickness was the thickness of the parietal pleura along the path of the needle as measured using US and was divided into thickness <3 mm and ≥3 mm. Pleural morphology was divided into the presence of pleural nodules/masses (Figure 1) and a non-nodular pleura. Biopsy factors included use of contrast agent, number of punctures, needle size, needle insertion angle (Figure 2), region of insertion, and precise location. The region reflected the distance from the diaphragm. If the distance from the costophrenic angle was <25 mm, we recorded biopsy at that angle. Location was divided into left or right thorax.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 (IBM, Armonk, NY, USA). We calculated diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). True-positive and true-negative cases were correct diagnoses. False-positive, false-negative, and inconclusive cases were incorrect diagnoses. Continuous variables were expressed as means with standard deviations, and categorical variables were expressed as frequencies or percentages. In univariate analyses, independent two-samples t-tests and the chi-square or Fisher’s exact test were used, as appropriate, to compare differences between groups. Multivariate logistic regression analysis was used to identify significant predictors of diagnostic success. P<0.05 was considered statistically significant.
Results
We performed 147 biopsies on 144 patients; seven patients were excluded because of loss to follow-up. Three patients underwent two procedures. Each biopsy procedure was recorded as an individual case. A total of 140 cases were ultimately included (105 males and 35 females). The average patient age was 55.3 years (range, 22–86 years).
The 140 pathological results of US-guided PCNBs revealed 26 malignant lesions, three cases of benign mesothelioma, specific infectious disease in 28 cases (27 cases of tuberculosis and one case of eosinophilic infection), 79 nonspecifically benign cases, and four cases for whom diagnosis failed (four cases of granulomatous inflammation). However the final diagnostic results were 43 malignant lesions (38 malignant metastatic tumors, five cases of malignant mesothelioma), five cases of benign mesothelioma, 40 cases of specific infectious disease (39 cases of tuberculosis and one case of eosinophilic infection), 49 nonspecifically benign cases, and three cases of granulomas that failed to resolve in terms of the final pathological inflammation. The pathological characteristics of initial US-guided PCNBs and final diagnoses are shown in Table 1.
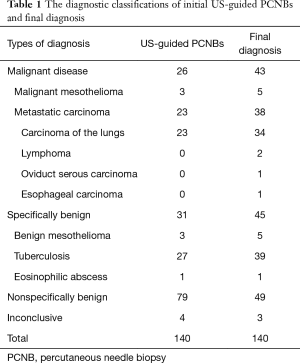
Full table
Of the 140 biopsies, 56 (40.0%) were true positives, 49 (35.0%) true negatives, 31 (22.1%) false negatives, 1 (0.7%) false positive, and 3 (2.1%) inconclusive. The only false-positive case was suspected squamous cell carcinoma on initial US-PCNB. However, the patient was eventually diagnosed thoracoscopically with tuberculous pleurisy. After antituberculosis treatment, the pleural fluid level in this patient did not increase during 12 months of follow-up. This case, false positive in terms of malignancy and false negative in terms of tuberculosis, was deemed to be a false-positive case overall. The 31 false-negative cases included 18 malignancies, 11 tuberculosis cases, and two benign mesotheliomas. Of the 18 malignant false negatives, seven were confirmed via standard pleural biopsy (SPB), five by analysis of transbronchoscopic biopsy specimens, two by histopathological analysis of a lung biopsy and thoracoscopic biopsy, three as metastases of extrapulmonary malignant tumors, and one patient was diagnosed with lymphoma in another hospital and died during follow-up. Six of the 11 tuberculosis cases were confirmed via SPB, three by histopathological analysis of transbronchoscopic biopsy specimens, one via laboratory testing of Mycobacterium tuberculosis, and one through a positive purified protein derivative (PPD) skin test (>10 mm) and clinical signs and symptoms. Two cases of benign mesothelioma were confirmed via SPB.
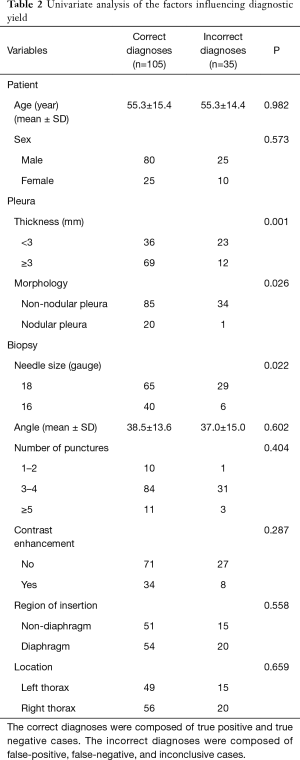
The overall accuracy of US-PCNB was 75.0%. The sensitivity, specificity, PPV, NPV in terms of malignant diagnosis were 58.1%, 99.0%, 96.2%, and 84.2%, respectively. The factors affecting diagnostic yield are shown in Table 2. On univariate analysis, variables affecting diagnostic accuracy of US-PCNB were the pleural thickness, morphology and needle size. Firstly, in the thickness of the pleura, there were 69 cases of thickened pleura (≥3 mm) and 36 of nonthickened pleura (<3 mm) in the correctly diagnosed group. The incorrectly diagnosed group contained 12 cases of thickened pleura (≥3 mm) and 23 cases of nonthickened pleura (<3 mm). Accuracy fell significantly (P=0.001) for patients with pleurae <3 mm in thickness (61.0%) compared to ≥3 mm in thickness (85.2%). And then, in the size of needle, in the correctly diagnosed group, 40 cases were performed by 16 G biopsy needle and 65 cases were performed by 18 G needle biopsy. In the incorrectly diagnosed group, six cases were performed by 16 G biopsy needle and 29 cases were performed by 18 G needle biopsy. The accuracy afforded by 18 G needles was significantly less than that afforded by 16 G needles (69.1% vs. 87.0%, P=0.022). The last, in the pleural morphology, the correctly diagnosed group consisted of 20 cases of pleural nodules and 85 cases without pleural nodules. But, there were 1 cases of pleural nodules and 34 cases without pleural nodules in the incorrectly diagnosed group. Compared to non-nodular pleurae, the diagnostic accuracies afforded by pleurae with nodules/masses was significantly higher (95.2% vs. 71.4%, P=0.026). Ultimately, multivariate logistic regression showed that pleural thickness [odds ratio (OR): 0.278, P=0.003] and needle size (OR: 0.291, P=0.018) independently predicted diagnostic accuracy (Table 3).
Full table

Full table
We included 140 cases, all of whom underwent diagnostic thoracocentesis and PCNB, and 79 of whom also later underwent Abrams biopsy. No severe intrathoracic hemorrhage was recorded; the principal complication was pneumothorax (four cases after thoracentesis + PCNB and 15 cases after thoracentesis + PCNB + Abrams).
Discussion
Our study shows that US-guided pleural cutting needle biopsy is effective with overall diagnostic accuracy in 75.0%, sensitivity and specificity of malignant diagnosis in 58.1% and 99.0%, respectively. In 1989 Macleod et al. were the first to introduce cutting needle biopsy using blind needles to explore large PEs (20). Soon afterward, ultrasound-guided Tru-cut PB was first reported by Chang et al. (15). Subsequent reports of US-guided PCNB proved the effectiveness of the technique; the overall diagnostic rate was 63% to 94%, and the sensitivity in terms of malignancy detection was 61% to 80% (12-15,21). In the present study, the diagnostic accuracy was 75.0%, within the range of prior publications. In terms of malignancy, our diagnostic sensitivity was 58.1%, slightly lower than the lowest reported sensitivity (14). We have an analysis of the following reasons. Firstly, we encountered 43 malignant cases, which is more than most previous studies. Chang et al. reported a 70% sensitivity, slightly higher than our value (15). But, Chang et al. included only 10 malignant cases (15). However, in another study, Metintas et al. (14) biopsied 49 patients with malignant pleural disease, the largest amount of malignant cases in previous studies, reported a diagnostic sensitivity of 61.2%, close to our 58.1%. Therefore, although the diagnostic sensitivity of this study is slightly lower than previous reports, we believe that the sensitivity of malignancy of this study is still highly representative because of the large number of malignant cases. Secondly, there are two sizes of biopsy needle (18 and 16 G) were used in this study. And smaller size biopsy needle (18 G) was used in 94 times, accounting for 67%. As has been said before, Metintas et al. (14) reported the number of cases and diagnostic sensitivity of malignancy similar to ours, but the diagnostic sensitivity in terms of malignancy is still slightly higher than our study. But what is different from us is that, in the Metintas et al. (14) study, all cases had used larger size biopsy needle (16 G) for biopsy. We think this one of the possible reasons why the diagnostic sensitivity of malignancy in our study is low compared to more recent studies. Finally, in this study, 19 of 43 malignant cases had no thickening of the pleura, which accounted for 44.2% of the malignant cases. The smaller size biopsy needle and thinner pleura may all affected the amount of material in biopsy and ultimately affected the diagnostic sensitivity of malignancy.
Although US-guided PCNB is valuable for diagnosing pleural disease, the failure rate ranges from 8% to 37% (12-15,21). Few previous reports have explored factors affecting diagnostic failures in large samples. To the best of our knowledge, ours is the first such study. We included 140 cases; divided them into those with correct and incorrect diagnoses; and explored relevant patient, pleural, and biopsy-related factors (Table 2). Univariate analyses showed that pleural thickness, pleural morphology, and needle size differed significantly between the two groups (P<0.05); ultimately, multivariate analyses confirmed that pleural thickness and needle size independently predicted diagnostic accuracy (Table 3).
Chang et al. and Metintas et al. reported diagnostic sensitivities in terms of malignancy of 70% and 61.2%, respectively, using 16 G cutting needles (14,15). However, using a larger diameter (14 G) needle, Koegelenberg et al. reported an overall diagnostic rate of 62.9% and a sensitivity of 66.7% in terms of malignancy detection (12). Heilo et al. found that the use of a larger cutting needle (14 vs. 18 G) afforded no additional diagnostic benefit (22). However, Adams et al. found that cutting needle biopsy was more sensitive than fine needle aspiration for diagnosing malignancies, including mesothelioma (23,24). John et al. found that increasing the needle caliber enhanced the diagnostic yield of percutaneous biopsy (25). Our findings, similar to those of Adams et al. And John et al., also showed that needle size significantly influences diagnostic accuracy. We found that the diagnostic accuracy of biopsy using a 16 G needle could reach 87.0%, significantly higher than the 69.1% associated with use of an 18 G needle (P=0.022). Adams et al. and Heilo et al. focused principally on malignancy or malignant pleural mesothelioma (22-24). We were concerned with all relevant diseases, including those for which diagnoses failed or were unclear. Our case distribution was malignancy in 30.7%, specifically benign in 32.1%. We are of the view that diagnostic accuracy is greatly aided by the use of a 16 G needle.
Thickening of the pleura or pleural nodules/masses evident on US or CT is an important sign of malignancy and an important indicator of PB (26-28). We routinely chose the thickest part of the pleura for biopsy. We found that the diagnostic accuracy of biopsy correlated significantly with pleural thickness. Diagnostic accuracy for cases with nonthickened pleurae (<3 mm) was lower than that for cases with thickened pleurae (≥3 mm) (61.0 vs. 85.2%, P<0.05). Niu et al. found no significant correlation between diagnostic accuracy when a pleural thickness of 15 mm was used to divide patients into two groups prior to CT-guided core needle biopsy of pleural lesions (16). However, in US-assisted PCNB, Metintas et al. found that the diagnostic sensitivity for cases of pleural thickness <1 cm was significantly lower than that for thickness ≥1 cm (42.9% vs. 80%) (14). It is interesting that Niu et al. included cases with pleural lesions/pleural thickening >5 mm maximum; PEs were not present in all cases (16). However, Adams et al. reported that PCNB of pleurae 0.2 to 0.5 cm in thickness afforded reliable histological diagnosis (23). In fact, 3 mm is often used for the standard to determine pleural thickening (29). In our clinic, we encounter many patients with unexplained PEs who require biopsy to allow us to plan treatment; such patients often have nonthickened pleurae (<3 mm). Thus, we compared cases with pleural thickness <3 and ≥3 mm and found that the diagnostic accuracy was 85.2% for the latter patients. In the work of Niu et al., all cases had a pleural thickness >5 mm, and the diagnostic accuracy of CT-guided PCNB of pleural lesions was 89.2%, close to our figure for those with pleural thickness ≥3 mm (16). This means that when the pleural thickness was ≥3 mm, US-guided PCNB afforded a high diagnostic rate. However, when the pleural thickness was <3 mm, the diagnostic accuracy was only 61.0%. In such cases, the lower diagnostic rate may be associated with smaller fragmented specimens. Thus, if patients with thin pleurae and unexplained PEs are not diagnosed via US-guided PCNB diagnosis, and other indicators or clinical symptoms suggest pleural positivity, follow-up biopsy or the use of other diagnostic methods is considerable (30,31).
Qureshi et al. found that US was effective at diagnosing malignant PEs; the suggestive signs were pleural thickness >10 mm, pleural nodularity, and diaphragm thickness >7 mm (27). Our diagnostic accuracy for cases with focal thickening or pleural nodules/masses was 95.2%. A total of 21 cases with focal pleural thickening or pleural nodules/masses were found in the present study, of which 17 (81%) were positive, including 10 malignancies, five tuberculosis cases, and two mesotheliomas; there were four negative cases (19%). Therefore, the presence of focal pleural thickening or nodules/masses is an important predictor of positivity even before biopsy. We found, in univariate analyses, that pleural nodules/masses were associated with a higher biopsy-indicated diagnostic rate than biopsy of non-nodular pleurae (95.2% vs. 71.4%, P=0.026). However, in multivariate analyses, the pleural morphology was not statistically significant. It is important to note that areas with pleural nodules/masses or focal thickening are usually the thickest regions. We encountered 21 cases of pleural nodules/masses and focal thickening. Of these, a pleural thickness ≥3 mm was evident in 17 cases; only four cases had a pleural thickness <3 mm, and one of these was a false negative. It should be noted that pleural morphology and thickness may be closely related; thus, in multivariate analyses, each may reduce the diagnostic accuracy afforded by the other. The diagnostic yield of cases with a pleural thickness ≥3 mm was significantly higher than that of cases with a pleural thickness <3 mm, so the choice of a thicker biopsy site improved the diagnostic rate. We selected pleural nodules/masses and regions of focal thickening for biopsy, which increased diagnostic accuracy. We believe that thorough scanning prior to biopsy is essential to locate regions of focal thickening or pleural nodules/masses and to biopsy those sites. The many causes of diagnostic failure include the biopsy of an unaffected area, inadequate tissue, or differences in pathological stages. Successful PB is reflected in not only specimen quantity but also quality. We suggest that thickened pleurae and pleural nodules/masses are so often positive because disease development is rather advanced or local disease is more obvious. Thus, the selection of such pleural regions for biopsy may improve diagnostic accuracy.
Our study has certain limitations: (I) the retrospective design creates a risk for selection bias. Because of the retrospective characteristics, the variables selected in our study were not comprehensive. For example, we could not get the accurate PE volume of all cases. The use of contrast agents, the selection of biopsy needles and the selection of puncture angles were not random, but subjective. Most patients were punctured three to five times to enhance diagnostic accuracy. The number of other punctures was less in this study; (II) not all biopsies were performed by the same operators, and we did not determine whether the operator affected diagnostic accuracy.
In conclusion, US-assisted PCNB is safe and affords a high diagnostic yield. Pleural thickness (<3 vs. ≥3 mm) and the size of the biopsy needle (18 vs. 16 G) were significantly correlated with the diagnostic yield.
Acknowledgements
Funding: This paper was supported by Science and Technology Planning Project of Guangdong Province, China (2017A020215062).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Scientific Research Ethics Review Committee of the First Affiliated Hospital of Guangzhou Medical University.
References
- Sahn SA, Heffner JE. Pleural fluid analysis. In: Light RW, Lee YC, editors. Textbook of Pleural Diseases. 2nd ed. London: Hodder Arnold; 2008:209-26.
- Du Rand I, Maskell N. Introduction and methods: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii1-3. [Crossref] [PubMed]
- Villena Garrido V, Ferrer Sancho J, Hernández Blasco L, et al. Diagnosis and treatment of pleural effusion. Arch Bronconeumol 2006;42:349-72. [PubMed]
- Hooper C, Lee YC, Maskell N. BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. [Crossref] [PubMed]
- Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015;70:995-7. [Crossref] [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [Crossref] [PubMed]
- Rahman NM, Gleeson FV. Image-guided pleural biopsy. Curr Opin Pulm Med 2008;14:331-6. [Crossref] [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. British Thoracic Society Pleural Disease Guideline Group. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [Crossref] [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [Crossref] [PubMed]
- Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology 2011;16:738-46. [Crossref] [PubMed]
- Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis 2015;7:1041-51. [PubMed]
- Koegelenberg CF, Bolliger CT, Theron J, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax 2010;65:857-62. [Crossref] [PubMed]
- Hallifax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014;146:1001-6. [Crossref] [PubMed]
- Metintas M, Yildirim H, Kaya T, et al. CT Scan-Guided Abrams' Needle Pleural Biopsy versus Ultrasound-Assisted Cutting Needle Pleural Biopsy for Diagnosis in Patients with Pleural Effusion: A Randomized, Controlled Trial. Respiration 2016;91:156-63. [Crossref] [PubMed]
- Chang DB, Yang PC, Luh KT, et al. Ultrasound-guided pleural biopsy with Tru-Cut needle. Chest 1991;100:1328-33. [Crossref] [PubMed]
- Niu XK, Bhetuwal A, Yang HF. CT-guided core needle biopsy of pleural lesions: evaluating diagnostic yield and associated complications. Korean J Radiol 2015;16:206-12. [Crossref] [PubMed]
- Benamore RE, Scott K, Richards CJ, et al. Image-guided pleural biopsy: diagnostic yield and complications. Clin Radiol 2006;61:700-5. [Crossref] [PubMed]
- Qureshi NR, Gleeson FV. Imaging of pleural disease. Clin Chest Med 2006;27:193-213. [Crossref] [PubMed]
- Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930-5. [Crossref] [PubMed]
- McLeod DT, Ternouth I, Nkanza N. Comparison of the Tru-cut biopsy needle with the Abrams punch for pleural biopsy. Thorax 1989;44:794-6. [Crossref] [PubMed]
- Stigt JA, Boers JE, Groen HJ. Analysis of "dry" mesothelioma with ultrasound guided biopsies. Lung Cancer 2012;78:229-33. [Crossref] [PubMed]
- Heilo A, Stenwig AE, Solheim OP. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology 1999;211:657-9. [Crossref] [PubMed]
- Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology 2001;219:510-4. [Crossref] [PubMed]
- Adams RF, Gray W, Davies RJ, et al. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest 2001;120:1798-802. [Crossref] [PubMed]
- Haaga JR. Clinical comparison of small-and large-caliber cutting needles for biopsy. Radiology 1983;146:665-7. [Crossref] [PubMed]
- Screaton NJ, Flower CD. Percutaneous needle biopsy of the pleura. Radiol Clin North Am 2000;38:293-301. [Crossref] [PubMed]
- Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009;64:139-43. [Crossref] [PubMed]
- Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. [Crossref] [PubMed]
- Koegelenberg CF, von Groote-Bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration 2012;84:337-50. [Crossref] [PubMed]
- Wang J, Zhou X, Xie X, et al. Combined ultrasound-guided cutting-needle biopsy and standard pleural biopsy for diagnosis of malignant pleural effusions. BMC Pulm Med 2016;16:155. [Crossref] [PubMed]
- Botana Rial M, Briones Gómez A, Ferrando Gabarda JR, et al. Tru-cut needle pleural biopsy and cytology as the initial procedure in the evaluation of pleural effusion. Arch Bronconeumol 2014;50:313-7. [PubMed]


