Successful HeartMate 3 implantation in isolated right heart failure—first in man experience of right heart configuration
Case presentation
In this article, we present the case of a 72-year-old male patient suffering from a severe form of singular isolated end-stage right heart failure based on arrhythmogenic right ventricular cardiomyopathy (ARVC), who was admitted to our clinic for acute cardiac decompensation. Echocardiography result revealed a dilated right atrium and ventricle (right ventricular end diastolic diameter, RVEDD: 59 mm) with a moderate tricuspid regurgitation owing to a dilated valve annulus of 48 mm. The left heart showed no pathological impairment: left ventricular end diastolic diameter (LVEDD) was 40 mm with a left ventricular ejection fraction (LVEF) of 50–55%. The patient also showed a progressive impairment of liver function by means of elevated levels of liver enzymes. Pulmonary artery embolism was excluded by computer tomography.
Despite maximum optimization of medical treatment, the patient showed no clinical improvement of right ventricular function. The patient displayed persisting symptoms including diminishing performance due to illness, severe peripheral edema, cardiac cachexia, ongoing noninvasive ventilation due to respiratory hypoxemia and a low cardiac output syndrome with a cardiac index of 1.4 L/min/m2. Due to these symptoms, along with the intermittent need of catecholamine support and the prior exhaustion of all therapeutic options (i.e., implantation of an implantable cardioverter device), the patient was evaluated for mechanical circulatory support of the right ventricle with a permanent ventricular assist device as an ultima ratio indication. After thoroughly interdisciplinary discussion as well as risk-benefit assessment, we decided to perform a singular right ventricular assist device (RVAD) implantation into the right atrium with outflow graft anastomosis to the pulmonary artery. Due to potentially low risk profile of HeartMate 3™-VAD (HM3, Abbott, North Chicago, IL, USA) with regard to thromboembolic occlusions, we decided to implant this LVAD type in the patient (1).
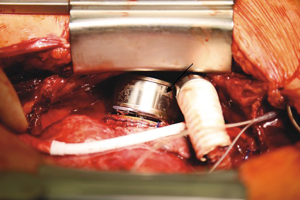
After full sternotomy, a widely enlarged right ventricle with a significantly decreased ejection function was observed, whereas the left ventricle showed no evident signs of functional impairment. We established cardiopulmonary bypass through cannulation of the ascending aorta and both vena cava superior and inferior. In order to avoid excessively deep penetration of the RVAD inflow cannula into the right atrium, we sutured several layers of felt pledgets onto the sewing ring of the ventricular assist device (Figure 1). Subsequently the sewing ring was positioned on the right atrium and incision with a complete inspection of the right atrium with regard to thrombi was performed on the beating heart without cardioplegia. All leads of the implantable cardioverter defibrillator (ICD) from the right heart were explanted. The RVAD was then placed in the sewing ring (Figures 2,3) and the outflow graft was anastomosed end-to-end to a smaller vascular prosthesis in order to reduce the diameter of the outflow graft from 14 to 10 mm. The 10 mm-prosthesis was then anastomosed end-to-side to the pulmonary artery. The driveline was tunneled subcutaneously above the rectus abdominis muscle. The duration of implantation was 216 minutes with a bypass time of 108 minutes.
After the procedure, the patient was transferred to the intensive care unit with minimal catecholamine support in good hemodynamic and respiratory conditions. RVAD parameters showed a flow rate of 4.2 L/min, speed of 5,000 rpm and motor power of 3.3 W. Postoperative echocardiography revealed an RVEDD of 4.15 cm and a mild tricuspid regurgitation. LVEDD was 4.8 cm with left ventricular ejection fraction of 50–55%.
Discussion
Albeit RVAD implantation has been performed in several cases, as an additional procedure after LVAD implantation, only few cases of singular RVAD implantation have been reported. This is on the one hand due to the low incidence of isolated right heart failure (2), and on the other hand due to technical challenges in implanting an RVAD (i.e., thinner myocardial wall, smaller ventricle leading to a higher incidence of suction events, thromboembolic events).
In this case report we report on a 72-year-old patient, who suffered from isolated right heart failure due to arrhythmogenic right ventricular cardiomyopathy, a rare inherited disease which is characterized by disruption of desmosomal proteins resulting in a replacement of cardiomyocytes by fibro-fatty tissue. This causes ventricular arrhythmias with an increased risk of sudden cardiac death; the gold standard in treatment of ARVC is therefore, the implantation of a cardioverter defibrillator, in addition to pharmacological treatment and catheter ablation of ventricular arrhythmias,
The patient showed severe symptoms of right heart failure (peripheral edema, cachexia, ongoing noninvasive ventilation due to respiratory hypoxaemia, hypostatic pneumonia) with a cardiac index of 1.4 L/min/m2. Optimization of medical treatment and antibiotic therapy of the pneumonia did not significantly alleviate the symptoms. Although there are clearly indications for LVAD implantation (e.g., NYHA IV, left ventricular ejection fraction <25%, progressive hepatic and/or renal failure secondary to hypoperfusion and to increased left ventricular filling pressure) (3,4), indications for the RVAD implantation are yet to be clearly defined. In this case, we adapted the indications of LVAD implantation: the patient was dependent on inotropic support, displayed severe symptoms despite optimal conservative and interventional therapy, progressive hepatic failure and had an impaired right ventricular function. After considering these factors and performing thorough multidisciplinary risk-benefit assessment, we decided to implant an RVAD as an ultima ratio indication. As it tends to show a lower risk profile regarding thromboembolic events, we selected the HeartMate 3™ for implantation (5-10).
The positioning of the ventricular assist device in right heart configuration remains the objective of discussion in the mechanical circulatory field. In this case, we decided to implant the RVAD into the left atrium, given the fact that it enables the right ventricle to recover and also that the right ventricle is associated with many trabecles, which may lead to higher rate of suction events that could more likely occur in the right ventricle. We also sutured several layers of felt pledgets onto the sewing ring of the HM3 in order to reduce the penetration depth of the inflow cannula into the right atrium, which could have led to suction events in the onset of the ventricular assist device. In this manner, we further reduced the risk of suction events, as it leads to more distance between the end of the inflow cannula and the interatrial septum. Nevertheless, implanting an assist device into the right atrium leads to a relief of the right ventricle, which could cause thrombi formation owing to changes in flow conditions.
In addition, we reduced the diameter of the outflow graft by end-to-end-anastomosis of a 10 mm graft onto the 14 mm outflow graft of the HM3. This resulted in a defined higher resistance in the outflow graft and therefore allowed consecutively higher rotational speed of the ventricular assist device, leading to a potentially lower risk of thrombus formation within the pump.
As mentioned earlier, the implantation of a RVAD leads to changes in the flow conditions of the left atrium (i.e., low-flow-setting to high-flow-setting). This in combination with the endothelialization of the ICD probes could have led to a detachment from cell debris with subsequent thrombotic events like pulmonary embolism or occlusion of the RVAD with a consecutive acute right heart failure. We therefore decided to completely remove the probes of the ICD and implant epicardial defibrillator probes, although we do not expect any arrhythmogenic complications in the future.
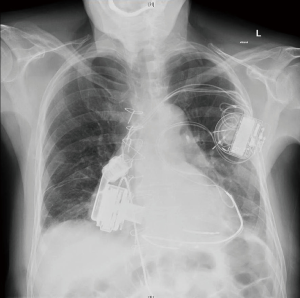
After implantation, the patient showed a distinct improvement of clinical symptoms. This has been confirmed by postoperative echo: the right ventricle was relieved (RVEDD 5.9 to 4.18 cm) and the left ventricle retained its ejection fraction (50–55%) with normalized LVEDD (4 cm). There were also no signs of pulmonary congestions in the chest X-ray.
Conclusions
In summary, we showed that the implantation of a singular RVAD is feasible as an ultima ratio in patients with symptomatic isolated right heart failure. Nevertheless, meticulous preoperative planning and surgical adaption of the ventricular assist device to the anatomical setting is crucial for the outcome of the patient. Furthermore, clear indications for the implantation of a singular RVAD should be evaluated.
Acknowledgements
Funding: This manuscript was kindly supported by a grant of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) through the project "KFO 311" (Principal Investigators: Prof. J Bauersachs, Prof. M Hoeper, Prof. JD Schmitto).
Footnote
Conflicts of Interest: JD Schmitto and G Dogan are consultants for Abbott. Other authors have no conflicts of interest to declare.
Informed Consent: This retrospective case study falls under the ethical approval and patient consent given with implantation at the Hannover Medical School.
References
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Mockel M, Searle J, Muller R, et al. Chief complaints in medical emergencies: do they relate to underlying disease and outcome? The Charité Emergency Medicine Study (CHARITEM). Eur J Emerg Med 2013;20:103-8. [Crossref] [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [Crossref] [PubMed]
- Ammirati E, Oliva F, Cannata A, et al. Current indications for heart transplantation and left ventricular assist device: a practical point of view. Eur J Intern Med 2014;25:422-9. [Crossref] [PubMed]
- Netuka I, Sood P, Pya Y, et al. Fully magnetically levitated left ventricular assist system for treating advanced hf: a multicenter study. J Am Coll Cardiol 2015;66:2579-89. [Crossref] [PubMed]
- Feldmann C, Chatterjee A, Hanke JS, et al. Novel centrifugal pump for heart failure patients: initial success and future challenges. J Thorac Dis 2017;9:1429-31. [Crossref] [PubMed]
- Feldmann C, Chatterjee A, Haverich A, et al. Left ventricular assist devices - a state of the art review. Adv Exp Med Biol 2018;1067:287-294. [Crossref] [PubMed]
- Ricklefs M, Hanke JS, Dogan G, et al. Less invasive surgical approaches for left ventricular assist device implantation. Semin Thorac Cardiovasc Surg 2018;30:1-6. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Hanke JS, Dogan G, Rojas SV, et al. First experiences with HeartMate 3 follow-up and adverse events. J Thorac Cardiovasc Surg 2017;154:173-8. [Crossref] [PubMed]