Bronchoscopic advances in the management of aerodigestive fistulas
Introduction
Aerodigestive fistula (ADF) is the formation of an abnormal tract between the airway and the digestive tract, most commonly the esophagus. Other terms used in the literature include broncho-esophageal, tracheo-esophageal or esophagorespiratory fistula.
ADF occurs in 5–15% of patients with esophageal or lung cancer, but may occur after trauma, or as a complication of surgery, stent placement and high endotracheal tube cuff pressure (1).
When occurring in the setting of malignancy such as lung or esophageal cancers, it is referred to as malignant ADF. This leads to significant morbidity and mortality as these patients die from pneumonia, sepsis and malnutrition within days to weeks if the fistula is left untreated (2-5).
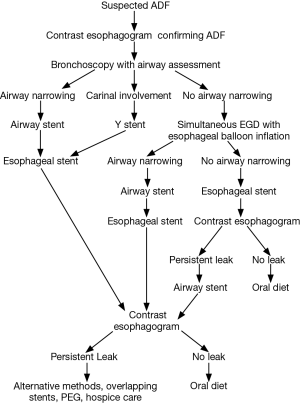
The diagnosis is usually suspected from the clinical symptoms and the medical history. Thin section computed tomography (CT) scan of the chest and a contrast esophagogram provide additional information on the location of the fistula. Bronchoscopy and endoscopy are necessary to confirm the diagnosis as well as to plan a therapeutic intervention. Visualization of small fistula can be improved by the administration of oral methylene blue prior to the bronchoscopy (6), or by the instillation of the methylene blue in the esophagus while performing simultaneous bronchoscopic and endoscopic procedures (7).
Different authors have described the location of the ADF. In a series of 63 patients, malignant ADF fistula most commonly occurred in the distal third of the trachea in 35%, the middle third in 29%, and the left main stem bronchus in 14% of the patients (8). In another series of 59 patients, malignant ADF occurred in the trachea in 50%, left main bronchus in 32%, and right main bronchus in 15% of the patients, while involving the main carina in 3% of the cases (9). In another series of 25 ADF, fistulas were localized to the proximal esophagus in 48%, the mid-esophagus in 20% and the distal esophagus in 24% of patients. In 8% (2/25) multiple fistulas were present (1). Although, most fistulae develop between the central airways and esophagus, some fistulae develop between the esophagus or stomach and the distal bronchi, small airways or lung parenchyma (2,3,10). In patients with esophageal resection for esophageal cancer, fistula formation can occur between the stomach that was pulled up into the chest and the airways (11).
The course of thoracic malignant diseases differs significantly from those of benign fistula, and thus management of ADF should follow a separate approach that takes in consideration the patients’ symptoms, quality of life and the estimated life expectancy.
For patients with acquired ADF of benign etiology, surgery may provide the best outcome as long as the patient is a good surgical candidate. This includes ADF division and primary repair, esophageal resection and reconstruction, esophageal diversion, suture closure of the fistula, pedicled tissue flap, segmental tracheal or bronchial resection (12,13).
The majority of patients with malignant ADF have either an advanced disease, metastasis or a high Eastern Cooperative Oncology Group (ECOG) score making them poor surgical candidates. Furthermore, ADF symptoms are distressing to patients and can disturb their quality of life later in the disease course. As such, palliation of these symptoms is recommended (14).
Bronchoscopic and endoscopic therapy is currently the main treatment for malignant ADF. In this review, we aim at providing an update regarding the latest advances of these various therapeutic approaches.
Endoscopic treatment
Goals of therapy
Patients with TE fistula typically presents with cough and dyspnea that is related to aspiration of their saliva and/or gastric contents. This lead to recurrent pulmonary infections and malnutrition with an increased mortality when no therapy is provided. Left untreated, patients with tracheoesophageal (TE) fistula have a median survival of few days to weeks (2-5,15).
The goals of treatment of the TE fistula is focused on palliation of the symptoms including cough, shortness of breath, dysphagia, decreasing the risk of aspiration as well as on improving the quality of life and survival (16).
Esophageal stenting only
Esophageal stenting using a self-expanding metallic stent (SEMS) has been used since 1980 (7). They are the most frequently used treatment modality (1). They are divided into partially covered stents and fully covered stents (Figure 1). While being equally effective at sealing the ADF, the overall clinical success rate at preventing aspiration range from 54–74% (1,17,18). Partially covered stents tend to embed in the esophageal wall making them less prone to migration but have a higher rate of tumor ingrowth. Fully covered stents have a higher rate of migration (17).
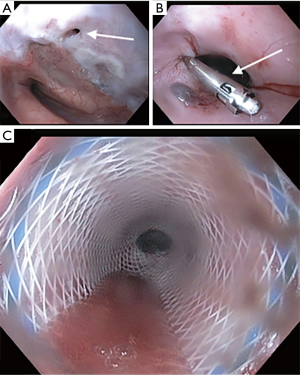
Stent related adverse events have been reported in around 30% to 37% of cases (18,19), and can be divided into early events (3%), such as esophageal perforation and tracheal compression, and late events (27%) such as stent migration, stent occlusion by tumor growth, upper gastrointestinal bleeding and stent induced fistula formation (18).
The most serious complication that can occur during esophageal stent placement is tracheal compression with airway compromise. This can occur immediately after placement of the esophageal stent or may be a delayed complication (18).
Airway stenting
Various types of airway stents have been used for palliation of the symptoms of ADF. These can be divided by type into partially or fully covered SEMS, silicone stents, and dynamic stents, or by shape into straight, L-shaped, I-shaped (cylindrical), hourglass-shaped or Y stent (Table 1).
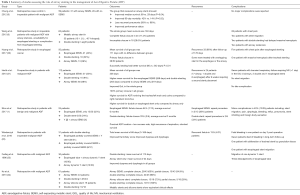
Full table
There is a paucity of trials evaluating the role of airway stent alone in the management of ADF (Figures 2,3). In a retrospective case control study, Chung et al. evaluated 31 patients who received airway SEMS only for ADF and compared them to 28 patients with similar baseline demographics who were managed conservatively only (9). Median survival, 90-day mortality and performance status were higher in the group that received an airway stent. Compared to the non-stented group’s median survival of 29 days, the group who received airway stent had a median survival of 69 days.
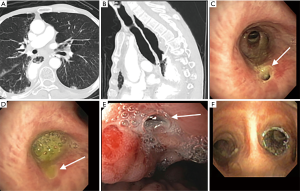
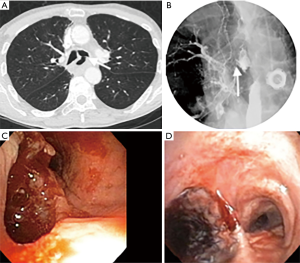
In another retrospective study done by Wang et al., 63 patients with malignant ADF who were deemed to be non-surgical candidates and who were either unsuitable for esophageal stent, had an airway stenosis or had a complication from esophageal stenting, received a customized metallic airway stent for malignant ADF. A total of 45 metallic Y stents, ten I-shaped stents and eight L-shaped stents were placed in the airway. Only 8 of these patients had additional esophageal stenting. Complete fistula closure was observed in 71.4% of the patients (45/63). There was an improvement in mean karnofsky score, and an overall mean survival of 163 days (8).
Huang et al. reported their outcome for 50 patients with malignant ADF who were managed with esophageal SEMS only (21/50), airway SEMS only (13/50) or received double stenting (16/50) (20). The mean survival was 171 days, with no significant difference between the three groups. Complete fistula closure was achieved in 28 of the 50 patients (56%), with recurrent ADF in 66% of the patients. There was no significant difference between the groups.
In 2010, Herth et al. reported one of the largest prospective trials, evaluating 112 patients with malignant ADF (21). Thirty-seven patients (33%) received an esophageal stent only, 65 patients (58%) received an airway SEMS only, and ten patients (9%) received double stenting. The mean survival was significantly longer in the group receiving an esophageal stent only or double stenting when compared to the group receiving an airway stent only (269 days vs. 253 and 219 days respectively). The ADF primary closure was 100% in all groups, but recurrence occurred in 24 patients (21%), and was more common in the airway stent only group (17 patients in the airway stent-only group compared to 6 in the esophageal SEMS-only and 1 in the double-stent group).
Double stenting
Double stenting (or parallel stenting) refer to the placement of an airway as well as an esophageal stent. Multiple studies have reported placement of an esophageal tube (23) or SEMS (1,20-22,24). The airway stenting was performed using SEMS (1,20-22,24), Silicone stent (1,22-24), or dynamic stent (23). In general, these studies are retrospective and involve a small number of patients. Few allow a direct comparison between single and double stenting (20,23,24).
Freitag et al. (23) published a retrospective study including 30 patients with malignant ADF who received either a Dynamic airway stent alone (12 patients) or combined airway and esophageal stenting (18 patients). Patients who received double stents had a longer survival compared with the airway stent only (110.2 vs. 23.8 days, P=0.0027). Fifty percent of the patients who received an airway stent only had persistent dysphagia, compared to 11% in the double-stenting group. Despite the limitations of the study, the authors concluded that double stenting may improve survival and quality of life compared to airway stenting alone.
More recently, Ke and his colleagues (24) reported their experience in the management of 62 patients with ADF who received airway and esophageal stenting.
Complete response was defined as no leakage of contrast medium after digital radiography and resolution of clinical symptoms without recurrence for more than two weeks. Partial response was defined as minor leakage of contrast medium with improvement of clinical symptoms that was maintained for more than 2 weeks. Failed treatment was defined as severe leakage of contrast medium with no improvement in clinical symptoms. Forty-three patients received an airway SEMS and 25 of the 43 patients received additional esophageal SEMS (double stenting). Complete fistula closure occurred in 65% (28/43) and partial closure in 35% (15/43) of the patients. When looking at the double stenting group only, 96% (24/25) achieved complete fistula closure. Similarly, they inserted 18 airway Dumont silicon stents in 18 patients. Ten of these patients received double stenting. Complete fistula closure was noted in 72% (13/18) and partial closure in 28% (5/18) of the patients. In the double stenting group, 100% (10/10) achieved complete fistula closure. The authors concluded that double stenting of the trachea and esophagus can achieve the best clinical benefit, and that metallic stents and silicone stents show equivalent clinical effects.
These results contrast with those reported by Huang et al. (20), who did not find a survival difference between patients who received single esophageal, single airway stent or double stenting.
Overlapping stent
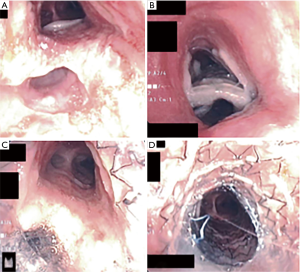
Overlapping refers to the insertion of a second stent in the same lumen (esophageal or airway) in which a previous stent exists, in order to allow sealing and occlusion of a previously stented fistula with incomplete closure. The two stents usually overlap over a small distance (Figure 4). Multiple studies have described this technique in the management of recurrent fistulae (20,21).
Approach to initial stent placement: esophageal versus airway stent
In general, an airway stent should be placed first when there is a concern for airway compromise, when there is a contraindication for esophageal stenting such as esophageal obstruction with inability to pass the wire beyond the obstruction, if the ADF is located in the upper third of the esophagus or if it is technically difficult to place a stent such in the case of gastro-tracheal fistula (Figure 2). In patients with sufficient nutrition that is provided via percutaneous endoscopic gastrostomy, an airway stent only may also be inserted (23).
If none of the above conditions are met, it appears that esophageal stent only or double stenting have an advantage over airway stenting alone. The American College of Chest Physicians gives a grade 1B recommendation for double stenting of the esophagus and bronchial tree or the esophagus alone with SEMS while managing patients with ADF (16).
Technical part
In general, a multidisciplinary approach by the endoscopist and the bronchoscopist is recommended to provide the optimal treatment of the ADF.
An airway evaluation to determine the degree of airway compromise should be done prior to the insertion of any esophageal stent. If there is visible airway narrowing, an airway stent should be placed first. In cases where there is suspicion of impending airway compromise after esophageal stent placement, simultaneous dual esophagostomy and bronchoscopy during which a balloon can be inflated in the esophagus to a diameter equal to that of the esophageal stent with direct bronchoscopic evaluation should be done (25). If significant airway narrowing develops, the airway should be stented first prior to esophageal stenting (26). If an airway stent is placed first, it should be followed by placement of an appropriately sized esophageal stent, since the outcome of double stenting appears better than single airway stent. If an esophageal stent is placed first it should be followed by an esophagogram in few days to detect persistent leak. If such a leak is present an airway stent should be placed (Figure 5).
The optimal stent length should be chosen to cover at least 2 cm beyond the proximal and distal margin of the lesion. The stent diameter should be at least 10–20% larger than the normal internal airway adjacent to the fistula (24).
The choice of the airway stent depends on the location of the fistula. Sites with close proximity to the carina may benefit from a customized silicone Y stent or dynamic airway stent placement, otherwise an appropriately sized SEMS or a silicone tube stent can be placed.
Metal stents can be placed with flexible bronchoscopy under direct or indirect visualization, using fluoroscopic guidance. Metal stent are composed of different metal alloys that gives them their radial force and metallic memory, which allows them to self-expend after deployment. They can adapt to different airway size and usually provide a good seal of the ADF. They can be fully covered or partially covered. Partially covered stents tend to embed in the mucosal wall making them less prone to migration, but have a higher rate of tumor ingrowth and granulation tissue (17,22). Fully covered stents have a higher rate of migration (17).
Silicone stents have similar radial force but require rigid bronchoscopy for insertion and tend to have a higher rate of migration. They are designed with outer studs to prevent migration and cannot conform to the airway anatomy, which may decrease their sealing effect.
Complications of airway stenting
The most serious complications of airway stenting are bleeding, enlarging or recurrent fistula, stent migration with recurrent aspiration, airway compromise and death. These events usually occur as a result of pressure necrosis and tissue erosion.
During long-term follow-up, recurrent fistula occurred in 13% to 66% of patients (20-22). Massive bleeding can occur immediately after stent placement (20,22), or at a later stage as reported by Wlodarczyk (7/31 patients) (22), and Wang (2/63 patients) (8).
In a review by Herth et al., respiratory failure requiring less than one day of mechanical ventilation occurred in 7 out of 112 patients (6%) after airway and esophageal stenting (21).
Minor complications such as chest pain, dysphagia, foreign body sensation, coughing and stent migration are frequent and can occur in up to 40% after stent placement (1).
Other techniques
Several other therapies have been reported in patients with TE fistulas. Despite the small number of patients evaluated, these techniques carry the potential of closing the fistula without the need for major interventions. However, one has to remember that technical success with all these methods does not always translate into clinical success (1).
Over-the-scope-clips (OTSC)
OTSC were introduced as a mean to close deep wall lesions (Figure 6). First reported in 2007 (28), the device uses a shape-memory allow (Nitinol). Much larger that the older through-the-scope (TTS) clips (29), they are deployed using an applicator integrated into the tip of an endoscope. The devices are available in several sizes (11, 12, and 14 mm), depth (3 and 6 mm) and type of teeth (30). Tissue suction is needed for successful application; hence, the fistula tissue needs to be soft and extensible (31). As a result, the clip is typically applied to the gastrointestinal side of the ADF. Overall, they appear to be more effective in treating perforations and leaks compared to fistulas (32), possibility related to the inability to completely approximate the borders that are frequently fibrotic. The technique can be used in combination with other modalities (1), such as esophageal/airway stenting and endoscopic sutures, potentially increasing its chances of clinical success.
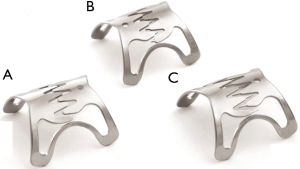
Another OTSC system called Padlock Clip (Aponos Medical, Kingston, NH, USA) was more recently introduced. It has the advantage of being located alongside the shaft of the endoscope, and therefore not requiring the working channel for deployment. Armellini et al. described its use in a small case series that included two patients with ADF (30). Technical success was obtained in all patients. Larger studies to determine its role in the management of such patients are needed.
Cardiac septal defect occluder
Another potential method of managing TE fistulas is the use of atrial septal defect (ASD) and ventricular septal defect (VSD) occluders. Made of nitinol, the dumbbell-shaped device is made of two discs of different diameters, connected by a thin waist. Deployed with the aid of fluoroscopy, each disc is positioned on one side of the fistula. Inflammatory response with granulation tissue and re-epithelialization over the device may occur. The clinical experience using these occluders in TE fistulas is limited (33). Device migration into the airway has been reported (34,35).
Septal button
ADF fistula closure has been attempted using silicone septal button that are designed to close nasal septal perforation. Schmitz et al. described the use of a septal button to successfully close a TE fistula after total laryngectomy in a case report (36). This resulted in improving the patient’s quality of life after failing standard therapies.
Fibrin glue
Closing TE fistula using fibrin glue has been described, mainly in the pediatric population. Made of thrombin and fibrinogen, fibrin glue has the advantage of immediate coagulation when calcium and factor XIII are added (through the conversion of fibrinogen to fibrin). If successful, it leads to rapid closure and scarring of the fistula. It can only be used in small fistulas, and one has to be careful about the possibility of major scope damage if the glue flows into the working channel (31). In a retrospective analysis that included 26 patients with esophageal fistulae or leakages, the application of fibrin glue was successful in nine cases (37). The remaining patients required other endoscopic/surgical interventions or continued to have persistent fistula/leakage. In the same study, 9.6% of the patients developed an abscess after the endoscopic intervention.
Polyglycolic acid (PGA) sheets
Another novel technique is the use of PGA sheets to promote the closure of TE fistulas. These are bioabsorbable polymers used during surgery to prevent delayed perforation by increasing the strength of sutures (29). Combined with fibrin glue, reports have described their use to enhance the closure of esophago-pulmonary fistula and esophago-bronchial fistula (38,39). More studies are needed to confirm their efficacy and apparent safety in this population.
Mesenchymal stem cells
Transplantation of mesenchymal stem cells is another interesting technique that could allow the closure of a fistula. Cells harvested from the bone marrow are injected into the defect, allowing it to close. In a recent case report, Petrella et al. used this technique to close a broncho-pleural fistula after a pneumonectomy (40). Even though we could not find any report on using this technique in the treatment of TE fistula, this could potentially be done for small defects.
Others
In a case of distal esophago-pleural fistula secondary to Boerhaave’s syndrome, Adler et al. used a combination of heat probe and endoscopic suturing to achieve a complete closure of the fistula (41). This technique could potentially be useful in the management of ADF. Other techniques include the use of biodegradable stent. However, the experience is limited to the pediatric literature (31).
Prognosis
Studies looking into prognostic features in patients with ADF suggest that achieving complete sealing of the ADF after stent therapy is associated with longer survival (80 vs. 242 days, P<0.01) (20).
It appears that the location of the ADF may affect survival as well. A lower survival was noticed in patients with right mainstem involvement when compared to ADF involving the trachea, left main bronchus and the carina (21). From the esophageal side, proximal esophageal location had the shortest survival (4.2 months) compared to distal location (7.8 months). Mid esophageal locations have an intermediate survival of 6 months (1).
Performance status (ECOG 3 or 4), pulmonary infection at the time of SEMS placement, and prior radiation therapy were also independent predictive factors associated with lower overall survival after stent placement (18).
Conclusions
Malignant ADF is a devastating condition with high morbidity and mortality. Airway and esophageal stenting remain the standard of care and is shown to significantly improve the quality of life and survival of these patients. Although more studies are available to guide our choice of therapy, high quality trials to establish the efficacy of each intervention are needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Silon B, Siddiqui AA, Taylor LJ, et al. Endoscopic Management of Esophagorespiratory Fistulas: A Multicenter Retrospective Study of Techniques and Outcomes. Dig Dis Sci 2017;62:424-31. [Crossref] [PubMed]
- Martini N, Goodner JT, D'Angio GJ, et al. Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg 1970;59:319-24. [PubMed]
- Burt M. Management of malignant esophagorespiratory fistula. Chest Surg Clin N Am 1996;6:765-76. [PubMed]
- Duranceau A, Jamieson GG. Malignant tracheoesophageal fistula. Ann Thorac Surg 1984;37:346-54. [Crossref] [PubMed]
- Gudovsky LM, Koroleva NS, Biryukov YB, et al. Tracheoesophageal fistulas. Ann Thorac Surg 1993;55:868-75. [Crossref] [PubMed]
- Shah A, Ost D, Eapen GA, et al. Diagnostic methylene blue test for stent covered tracheoesophageal fistula. Am J Respir Crit Care Med 2012;185. [Crossref] [PubMed]
- Porumb V, Cozorici A, Andrese E, et al. Palliative Treatment of Malignant Esophagopulmonary Fistulas with Covered Self-Expandable Metallic Stents (SEMSs). A Single Center Experience. Rev Med Chir Soc Med Nat Iasi 2015;119:425-30. [PubMed]
- Wang H, Tao M, Zhang N, et al. Airway Covered Metallic Stent Based on Different Fistula Location and Size in Malignant Tracheoesophageal Fistula. Am J Med Sci 2015;350:364-8. [Crossref] [PubMed]
- Chung FT, Lin HC, Chou CL, et al. Airway ultraflex stenting in esophageal cancer with esophagorespiratory fistula. Am J Med Sci 2012;344:105-9. [Crossref] [PubMed]
- Burt M, Diehl W, Martini N, et al. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg 1991;52:1222-8; discussion 1228-9. [Crossref] [PubMed]
- Li YD, Li MH, Han XW, et al. Gastrotracheal and gastrobronchial fistulas: management with covered expandable metallic stents. J Vasc Interv Radiol 2006;17:1649-56. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC, Wain JC, et al. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 1991;52:759-65. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [Crossref] [PubMed]
- Kvale PA, Simoff M, Prakash UB. Lung cancer. Palliative care. Chest 2003;123:284S-311S. [Crossref] [PubMed]
- Gschossmann JM, Bonner JA, Foote RL, et al. Malignant tracheoesophageal fistula in patients with esophageal cancer. Cancer 1993;72:1513-21. [Crossref] [PubMed]
- Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e455S-e497S.
- van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol 2012;12:19. [Crossref] [PubMed]
- Ribeiro MSI, da Costa Martins B, Simas de Lima M, et al. Self-expandable metal stent for malignant esophagorespiratory fistula: predictive factors associated with clinical failure. Gastrointest Endosc 2018;87:390-6. [Crossref] [PubMed]
- Kim PH, Kim KY, Song HY, et al. Self-Expandable Metal Stent Use to Palliate Malignant Esophagorespiratory Fistulas in 88 Patients. J Vasc Interv Radiol 2018;29:320-7. [Crossref] [PubMed]
- Huang PM, Lee JM. Are single or dual luminal covered expandable metallic stents suitable for esophageal squamous cell carcinoma with esophago-airway fistula? Surg Endosc 2017;31:1148-55. [Crossref] [PubMed]
- Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36:1370-4. [Crossref] [PubMed]
- Włodarczyk J, Kużdżał J. Double stenting for malignant oesophago-respiratory fistula. Wideochir Inne Tech Maloinwazyjne 2016;11:214-21. [Crossref] [PubMed]
- Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest 1996;110:1155-60. [Crossref] [PubMed]
- Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis 2015;7:S389-97. [PubMed]
- Zori AG, Jantz MA, Forsmark CE, et al. Simultaneous dual scope endotherapy of esophago-airway fistulas and obstructions. Dis Esophagus 2014;27:428-34. [Crossref] [PubMed]
- Lanfranco AR, Haas A, Sterman D. Airway stenting for malignant aerodigestive fistulae: A critical review of the literature and treatment recommendations. Tech Gastrointest Endosc 2009;11:118-26. [Crossref]
- Kothari TH, Haber G, Sonpal N, et al. The Over-the-Scope Clip System – A Novel Technique for Sastrocutaneous Fistula closure: The first North American Experience. Can J Gastroenterol 2012;26:193-5. [Crossref] [PubMed]
- Kirschniak A, Kratt T, Stuker D, et al. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc 2007;66:162-7. [Crossref] [PubMed]
- Adler DG. Endoscopic Management of Esophagorespiratory Fistulas. Pract Gastroenterol 2017. Available online: https://www.practicalgastro.com/pdf/September17/Endoscopic-Management-of-Esophagorespiratory-Fistulas.pdf
- Armellini E, Crino SF, Orsello M, et al. Novel endoscopic over-the-scope clip system. World J Gastroenterol 2015;21:13587-92. [Crossref] [PubMed]
- Zhou C, Hu Y, Xiao Y, et al. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis 2017;11:173-80. [Crossref] [PubMed]
- Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014;80:610-22. [Crossref] [PubMed]
- Scordamaglio PR, Tedde ML, Minamoto H, et al. Endoscopic treatment of tracheobronchial tree fistulas using atrial septal defect occluders: preliminary results. J Bras Pneumol 2009;35:1156-60. [Crossref] [PubMed]
- Coppola F, Boccuzzi G, Rossi G, et al. Cardiac septal umbrella for closure of a tracheoesophageal fistula. Endoscopy 2010;42 Suppl 2:E318-9. [Crossref] [PubMed]
- Miller PE, Arias S, Lee H, et al. Complications associated with the use of the amplatzer device for the management of tracheoesophageal fistula. Ann Am Thorac Soc 2014;11:1507-9. [Crossref] [PubMed]
- Schmitz S, Van Damme JP, Hamoir M. A simple technique for closure of persistent tracheoesophageal fistula after total laryngectomy. Otolaryngol Head Neck Surg 2009;140:601-3. [Crossref] [PubMed]
- Lippert E, Klebl FH, Schweller F, et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis 2011;26:303-11. [Crossref] [PubMed]
- Matsuura N, Hanaoka N, Ishihara R, et al. Polyglycolic acid sheets for closure of refractory esophago-pulmonary fistula after esophagectomy. Endoscopy 2016;48 Suppl 1 UCTN:E78-9.
- Kinoshita S, Nishizawa T, Hisamatsu T, et al. Polyglycolic acid sheet for closure of esophagobronchial fistula in a patient with Behcet's disease. Gastrointest Endosc 2017;85:1094-6. [Crossref] [PubMed]
- Petrella F, Spaggiari L, Acocella F, et al. Airway fistula closure after stem-cell infusion. N Engl J Med 2015;372:96-7. [Crossref] [PubMed]
- Adler DG, McAfee M, Gostout CJ. Closure of an esophagopleural fistula by using fistula tract coagulation and an endoscopic suturing device. Gastrointest Endosc 2001;54:652-3. [Crossref] [PubMed]