Surgical resection of a giant polycystic seminoma of the mediastinum
Introduction
Mediastinal germ cell tumors consist of only 10–15% of all mediastinal tumors, while mediastinal seminomas account for approximately 25–40% of all primary malignant mediastinal germ cell tumors (1,2). These are slow-growing tumors that typically manifest as a solid, lobulated mass on a computed tomography (CT) scan (3); no specific serum tumor markers exist on mediastinal seminomas. Here, we describe a patient with a giant polycystic seminoma that was diagnosed on pathologic examination after surgical resection.
Case presentation
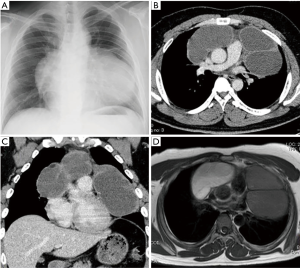
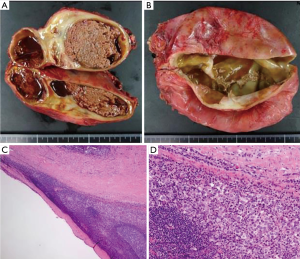
A 29-year-old man was referred to our hospital with a history of a 1-year worsening cough and shortness of breath. Physical examination showed diminished breath sounds in the left precordium. Chest radiograph imaging demonstrated a huge mass adjacent to the cardiac shadow (Figure 1A). The chest CT scan revealed large polycystic tumors in the anterior mediastinum near both thoracic cavities without findings of invasion of the lungs or great vessels (Figure 1B,C). Chest magnetic resonance imaging scan showed a large multilocular cystic tumor. The right side of the tumor had a high intensity, whereas the left side of the tumor showed a low intensity on T1-weighted imaging (Figure 1D). Laboratory examinations of serum tumor markers, such as lactate dehydrogenase (LDH), carcinoembryonic antigen, alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (βhCG), soluble interleukin-2 receptor, and anti-acetylcholine receptor antibody levels, were within normal limits. Ultrasonography showed that the testicles were normal. We did not perform a percutaneous biopsy because cyst walls were thin to access percutaneously. We decided to perform surgical resection because tumor removal seemed to be needed to improve his symptoms, and thymic tumors, such as cystic thymoma and thymic cysts were suspected. Surgical resection of the tumors was performed through median sternotomy. The left side of the tumor slightly adhered to the left upper lobe; however, no invasion to surrounding structures, including phrenic nerve and pericardium, was identified. Macroscopic en bloc resection was achieved. The resected specimens were polycystic masses with solid components; the right side of the tumor measured 13×10×4 cm3, and the left side of the tumor measured 13×13×4 cm3 (Figure 2A,B). Pathologic examination showed that the thick parts of cyst walls were composed of tumor cells with round to oval, small, mature lymphocytes, and squamous epithelium with scattered foci of residual thymic parenchyma (Figure 2C,D). The tumor cells had abundant clear cytoplasm that glowed. The surgical margins were negative. Immunohistochemical staining showed that the tumor cells were positive for placental alkaline phosphatase, c-kit, and D2-40, but negative for AE1/AE3, AFP, and βhCG. These findings were consistent with seminoma. The possibility that seminoma developed due to thymic cysts was suggested because of the presence of scattered thymic cells on the cyst walls. The patient was discharged seven days post-operatively without any complications or symptoms. After the patient was discussed with urologists, he has been followed without any adjuvant therapy. The patient has been alive for 18 months with no evidence of recurrence.
Comments
Mediastinal germ cell tumors account for only 10–15% of mediastinal tumors and mediastinal seminomas account for approximately 25–40% of all primary malignant mediastinal germ cell tumors (1,2). Thus, mediastinal seminomas consist of approximately 3–5% of malignant mediastinal tumors, and are rare malignant germ cell tumor of the mediastinum.
Mediastinal seminomas typically manifest as a solid, lobulated mass on a CT scan. Thymic cyst, cystic thymoma, thymic cancer, mature cystic teratoma, cystic seminoma, and bronchogenic cyst manifest as a cystic mass of the mediastinum on CT scan; cystic seminoma is rare in mediastinal tumors with these radiologic findings (4). Some patients with mediastinal seminomas may show a mild elevation of βhCG, and elevation of serum AFP levels indicates the presence of nonseminomatous tumor (2). Although elevated serum LDH is seen in patients with advanced seminoma and elevated βhCG is usually an independent adverse prognostic factor for survival in adults with seminoma, there are no specific serum tumor markers and image findings for early-stage mediastinal seminoma (1,5). Thus, patients with cystic seminoma have often undergone surgical resection and been diagnosed based on the postoperative pathological examination (6). Fine needle aspiration cytology could help a preoperative diagnosis for cisplatin-based chemotherapy as the first-line of therapy (7). However, in the present case, a percutaneous needle biopsy was deemed impossible because of small thinning components around large polycystic masses. The components of seminoma cells were small, even on the pathological examination. Moreover, we thought that surgical tumor resection was required for this patient to improve symptoms due to intrathoracic organs compression by the tumors.
The initial treatment for locally advanced and bulky mediastinal seminoma is currently cisplatin-based chemotherapy with or without supradiaphragmatic radiotherapy (2). However, a favorable prognosis after complete resection without adjuvant therapy for patients with mediastinal cystic seminoma was reported, especially for young patients (8). Thus, we have followed up the patients without any adjuvant therapy, and no signs of recurrence have been identified for 18 months after surgical resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Rosado-de-Christenson ML, Templeton PA, Moran CA. From the archives of the AFIP. Mediastinal germ cell tumors: radiologic and pathologic correlation. Radiographics 1992;12:1013-30. [Crossref] [PubMed]
- Cameron RB, Loehrer PJ, Thomas CR. Neoplasms of the Mediastinum. IN: DeVita VT, Lawrence TS, Rosenberg SA. editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 9th edition. Philadelphia: Lippincott Williams and Wilkins, a Wolters Kluwer, 2011:871-81.
- Strollo DC, Rosado-de-Christenson ML. Primary mediastinal malignant germ cell neoplasms: imaging features. Chest Surg Clin N Am 2002;12:645-58. [Crossref] [PubMed]
- Kim JH, Goo JM, Lee HJ, et al. Cystic tumors in the anterior mediastinum. Radiologic-pathological correlation. J Comput Assist Tomogr 2003;27:714-23. [Crossref] [PubMed]
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864-73. [Crossref] [PubMed]
- Lin MW, Chang YL, Wu CT, et al. Video-assisted thoracoscopic surgery for a cystic seminoma of the mediastinum. Ann Thorac Surg 2010;90:2041-4. [Crossref] [PubMed]
- Silverman JF, Olson PR, Dabbs DJ, et al. Fine-needle aspiration cytology of a mediastinal seminoma associated with multilocular thymic cyst. Diagn Cytopathol 1999;20:224-8. [Crossref] [PubMed]
- Hata Y, Isobe K, Sato F, et al. Anterior mediastinal cystic seminoma. Thoracic Cancer 2013;4:75-8. [Crossref] [PubMed]


