Efficacy and immune activation of ipilimumab in early-stage lung cancer patients
Immune checkpoint inhibitors, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody and programmed death-1 (PD-1) antibody, improve the prognosis in several types of cancer patients, and they modulate the effector T-cell activation, proliferation and function (1). Ipilimumab is a monoclonal antibody targeting CTLA-4 (2). Generally, B7 receptors, represented by B7.1 (CD80) and B7.2 (CD86), are expressed on antigen-presenting cells (APCs), and T-cells are activated by the binding of B7.1/B7.2 and CD28, which is the principal co-stimulating receptor of T-cells (2). CTLA-4 preferentially binds B7.1/B7.2 (CD80/CD86) over CD28 because of a stronger affinity of CTLA-4 for B7.1/B7.2 (CD80/CD86) than CD28 on APCs, and the connection between B7 receptors and CTLA-4 downregulates the proliferation and activation of T-cells (2), resulting in the enhancement of the immune response against the tumor.
The recent article by Yi et al. (3) reported that neoadjuvant chemotherapy due to ipilimumab in addition to carboplatin/paclitaxel showed additive efficacy compared with carboplatin/paclitaxel chemotherapy in early-stage non-small cell lung cancer (NSCLC). Several reports of the efficacy of ipilimumab in lung cancer have been published, and the concurrent combination therapy of carboplatin/paclitaxel and ipilimumab did not improve the progression-free survival (PFS) compared with carboplatin/paclitaxel chemotherapy only. However, phased ipilimumab did improve the immune-related PFS versus chemotherapy only (HR =0.64; P=0.03) in a phase II study of lung cancer (4,5). In the study by Yi et al. (3), a phased ipilimumab and carboplatin/paclitaxel regimen was selected based on previous studies (2,4). Recently, however, the carboplatin/paclitaxel plus ipilimumab regimen used in a phase III study failed to prolong the overall survival (OS) compared with chemotherapy alone in patients with advanced squamous cell carcinoma (13.4 vs. 12.4 months; P=0.25) (6); therefore, ipilimumab in addition to carboplatin/paclitaxel might not be a promising regimen. Of note: combination therapy of nivolumab and ipilimumab in the first-line treatment of NSCLC patients showed a higher objective response rate (43%) than nivolumab only (23%) and showed tolerable safety in CheckMate 012 (1), especially in patients with high levels of tumor PD-L1 [tumor proportion score (TPS)].
The characteristics of ongoing clinical trials are shown in Table 1. Many of these ongoing clinical trials evaluate the efficacy and safety of the combination with nivolumab/pembrolizumab and ipilimumab (Table 1). In addition, the efficacy of monotherapy with ipilimumab has not been shown in lung cancer patients. Therefore, we want to determine the influences of the combination therapy of nivolumab/pembrolizumab and ipilimumab for the immune response as below.
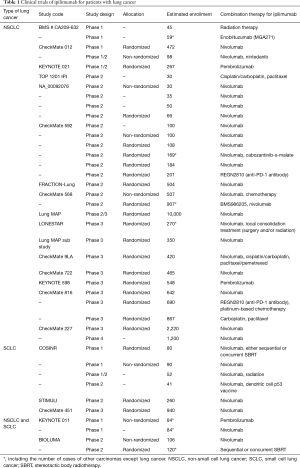
Full table
Curran et al. reported that anti-CTLA-4 inhibitor leads to an increase in CD4+/CD8+ effector T cells in mice (7), and Kitano et al. also reported that it induces and enhances the cytotoxic function of CD4+ T cells, in addition to CD8+ T cells, specialty to cancer antigens in melanoma patients (8). Thus, CTLA-4 blockade induces an expansion of CD4 T cells in addition to engaging specific subsets of exhausted-like CD8 T cells (9). Yi et al. (3) demonstrated the increased activation of peripheral blood CD4+ and CD8+ T cells after ipilimumab therapy in lung cancer patients. Their results suggest that ipilimumab affects not only CD8 but also CD4 T cells, especially CD4 T cells expressing the activation markers inducible T-cell co-stimulator (ICOS), human leukocyte antigen-antigen D related (HLA-DR), CTLA-4, and PD-1, among peripheral blood mononuclear cells (PBMCs) at several time points after ipilimumab administration. In addition, blood regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs) as well as the PD-1 expression on CD8 T cells were not influenced by ipilimumab treatment, suggesting that ipilimumab treatment itself may not be sufficient but is nevertheless necessary for a therapeutic immune effect in patients with NSCLC. These results also suggest that the stratification of lymphocyte activation was not associated with the overall response to ipilimumab, although it is reported that an increase in ICOS+ T cells was associated with a good prognosis (10).
The reason why the activation of these factors was not associated with a positive OS may be due to the small sample size and other immunotherapeutic effects (3). Therefore, large multicenter trials with a large number of patients are necessary to clarify this association. In addition, no correlation was noted between the degree of PD-L1 expression on the tumor cells and the therapeutic effects of nivolumab in patients with squamous cell carcinoma (11); however, the PD-L1 expression on immune cells locally infiltrating around the tumor was correlated more strongly with the therapeutic effects of nivolumab than the expression on the tumor cells themselves (12).
Yi et al. (3) suggested that the correlation between the activation of CD4+/CD8+ T cells in tumor-infiltrating lymphocytes (TILs) and the prolongation of the OS may be the next question that should be explored. Recent understanding of the correlation of TIM-3 and PD-1 antibody in lung cancer immunity, immunological changes and clinical impacts induced by an administration of ipilimumab are what we now want to know, in addition to the changes of PBMCs after surgical resection of cancer.
CTLA-4 is also expressed on the surface of Tregs (2), which are a key factor in ipilimumab treatment. CD4+CD25+ Tregs suppress the antitumor immune response in animal models (13), and the tumor shrinkage effect depends on the removal of Tregs within the tumor by antibody-dependent cellular cytotoxicity (ADCC) (14). Yi et al. (3) clarified that ipilimumab had little or no effect on the frequencies of circulating Tregs, and data on the significance of the frequencies of circulating Tregs are insufficient at present. Further investigations of the response of Tregs in tumor tissues are necessary to elucidate the antitumor effects of ipilimumab.
Yi et al. (3) showed that the activation of CD8+ T cells can be CD28+-dependent, but the CD28 expression did not change in response to chemotherapy or ipilimumab treatment; however, these findings cannot explain the clinical efficacy of all immunotherapeutic agents. In addition, Kitano et al. reported that low levels of MDSCs (<14.9%) were associated with a significantly prolonged OS (15), and two patients with high MDSCs (≥15%) showed a poor response in the study of Yi et al. (3). The authors (3) partially explain the mechanism underlying the activation and maintenance of peripheral blood/tumor-infiltrating T lymphocytes in early-stage NSCLC patients, but further understanding of the sequential mechanism underlying the clinically-effective immunological responses induced by a combination of immunotherapeutic agents in these patients is needed. In addition, no useful biomarkers have yet been established for estimating the clinical efficacy of ipilimumab treatment in lung cancer patients. As such, further investigations of good biomarkers, including CD28 and MDSCs, are needed, as ipilimumab treatment requires a takes long time to achieve antitumor effects.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Genova C, Rijavec E, Barletta G, et al. Ipilimumab (MDX-010) in the treatment of non-small cell lung cancer. Expert Opin Biol Ther 2012;12:939-48. [Crossref] [PubMed]
- Yi JS, Ready N, Healy P, et al. Immune Activation in Early-Stage Non-Small Cell Lung Cancer Patients Receiving Neoadjuvant Chemotherapy Plus Ipilimumab. Clin Cancer Res 2017;23:7474-82. [Crossref] [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Govindan R, Szczesna A, Ahn MJ, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3449-57. [Crossref] [PubMed]
- Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275-80. [Crossref] [PubMed]
- Kitano S, Tsuji T, Liu C, et al. Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res 2013;1:235-44. [Crossref] [PubMed]
- Wei SC, Levine JH, Cogdill AP, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017;170:1120-33.e17. [Crossref] [PubMed]
- Di Giacomo AM, Calabrò L, Danielli R, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 2013;62:1021-8. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999;163:5211-8. [PubMed]
- Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695-710. [Crossref] [PubMed]
- Kitano S, Postow MA, Ziegler CG, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res 2014;2:812-21. [Crossref] [PubMed]


