Thoracoscopic segmentectomy for small-sized peripheral lung cancer
Introduction
Since 1960, the standard surgical procedure for early stage lung cancer has been lobectomy with mediastinal lymph node dissection (1). However, recently, early detection of peripheral small pulmonary nodules such as ground glass nodule (GGN) has been increasing due to recent advancement of low dose computed tomography (CT) screening. Sub-lobar resection, such as segmentectomy or wedge resection, must be performed in patients with small-sized peripheral lung tumors on preoperative CT. Segmentectomy has some advantages compared to wedge resection regarding obtaining surgical margin and assessment of hilar lymph nodes. However, anatomical segmentectomy, because of the need to identify variances in pulmonary vessels and the intersegmental line during planning for lung anatomical segmentectomy, is a technically more complicated operative procedure than lobectomy. In addition, in thoracoscopic procedure, we cannot observe the intrathoracic structure under three-dimensional field of vision. We retrospectively reviewed our indication, surgical procedure, and results of thoracoscopic segmentectomy (TS-S).
Methods
This study was approved by Cancer Institute Hospital Institutional Review Board of Clinical Research, and the need for informed consent from patients was waived because of its retrospective design.
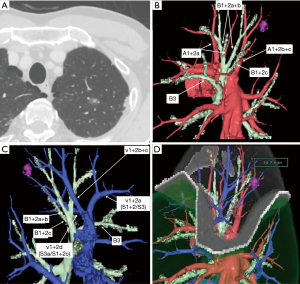
From April 2008 to December 2016, 191 patients who underwent TS-S for small-sized peripheral lung cancer were reviewed retrospectively. Multidetector-row CT (MDCT) was routinely performed preoperatively. Using MDCT data, we performed three-dimensional simulation of the vascular structures, segmental bronchus, and tumor using simulation software (Volume Analyzer Synapse Vincent; Fujifilm Medical Systems, Tokyo, Japan). The simulation workstation allowed us to create a virtual intersegmental plane after plotting the dominant segmental pulmonary artery and to measure the surgical margin from the tumor to the closest intersegmental plane, thus allowing proper preoperative planning for all patients (Figure 1). Our intentional indication of TS-S is peripheral radiologically noninvasive lung cancer whose tumor size is less than 2 cm in size with consolidation to tumor (C/T) ratio less than 0.5. Most these lesions have no angiolymphatic invasion. So, if surgical margin is enough, these lesions are usually indication for performing a wedge resection. However, if the lesion is located deep from the lung surface, to secure a sufficient surgical margin may be difficult. In such situation, we select to conduct segmentectomy. In contrast, compromised indication is radiologically invasive lung cancer (C/T ration more than 0.5) which we can keep sufficient surgical margin in patient with previous history of contralateral lobectomy, poor pulmonary reserve, and octogenarians.
Tumor stage was described according to the TNM classification of 2009 (2).
Our Technique of TS-S
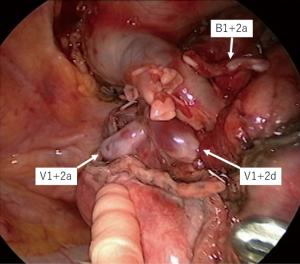
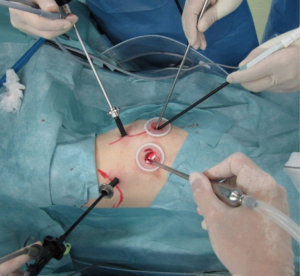
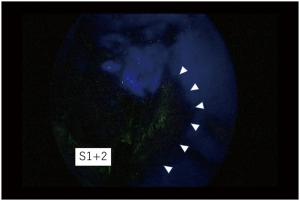
The surgical procedure of TS-S was performed via a 2.5-cm utility incision at the posterior axillary line at the 5th intercostal space using endoscopic instruments and traditional instruments for sharp dissection. The latissimus dorsi was left intact and the serratus anterior was split and the intercostal muscles minimally divided about 3 cm to remove the resected specimens. A 15-mm incision was created at the anterior axillary line at the 4th intercostal space for the second assistant. Two 7-mm ports were placed at the center of the 3rd intercostal space for the camera and posteriorly at the 3rd intercostal space for the operator’s left hand. Two silicone rubber instruments (Lap Protector™ Minimini; Hakko Co, Ltd., Tokyo, Japan) were applied to maintain the two wounds open (Figure 2). The operation was performed entirely via thoracoscopic visualization and the role of each port was fixed. Dissection and division of segmental artery and bronchi in TS-S was same as that in lobectomy. To confirm the intersegmental plane, in some cases, we used inflation deflation line which was made by butterfly needle inflation to the target segment. Recently, we performed indocyanine green (ICG) fluorescence navigated method to visualize the intersegmental border (Figure 3). And finally, lung parenchyma was divided using electrocautery or endoscopic staples along intersegmental border (Figure 4). As a rule, we tried to keep the surgical margin larger than size of tumor or 1 cm.
Patients were followed until their death or up to the time of analysis in January 2018. Actuarial survival curves were constructed by the Kaplan-Meier methods, using Statview 5.0 software (SAS Institute, Cary, NC, USA).
Results
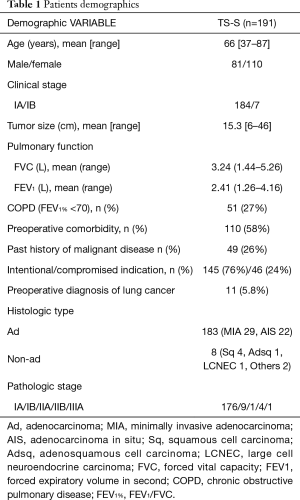
Patient characteristics are shown in Table 1. The study patients comprised 81 men and 110 women (mean age, 66 years; range, 37–87 years). Preoperative clinical stage of the disease determined that 184 patients had stage IA, and 7 patients had IB on preoperative CT, with normal (<1 cm) mediastinal lymph nodes. Preoperative PET scan was performed in 32 patients (17%) to evaluate the staging. Average tumor size on preoperative CT was 15.3 mm (range, 6–46 mm). Four patients with a big tumor more than 3 cm in size were performed TS-S (3 upper division tri-segmentectomies, 1 lingular segmentectomy) as a compromised indication. Average pulmonary function as measured by forced expiratory volume in 1 s (FEV1) was 2.41 L (range, 1.26–4.16 L). One hundred and ten of 191 patients (58%) had comorbidity. Forty-nine patients (26%) had previously been treated for malignant disease, including 14 with lung cancer, 8 with gastric cancer, and 6 prostate cancer. TS-S was performed as a compromised indication in 46 patients (24%; past history of lobectomy 13, synchronous multiple lung cancer 10, octogenarian 8, poor pulmonary reserve 4, others 11). Preoperative diagnosis of lung cancer was confirmed in 11 patients (5.8%) by bronchoscope or CT guided needle biopsy. Almost cases were difficult to confirm the preoperative diagnosis, because there were many tiny ground-glass nodules in this cohort. Final histopathologic diagnosis were 183 adenocarcinomas (96%) including 29 minimally invasive adenocarcinomas (MIA) and 22 adenocarcinomas in situ (AIS), and 8 other types of carcinoma. The pathologic stage of lung cancer was classified as IA in 176 patients (92%), IB in 9 patients, IIA in 1 patient, IIB in 4 patients, and IIIA in 1 patient. No patients received postoperative adjuvant chemotherapy. All patients are still being followed up by CT and/ or PET scan every 6 or 8 months at our institution.
Full table
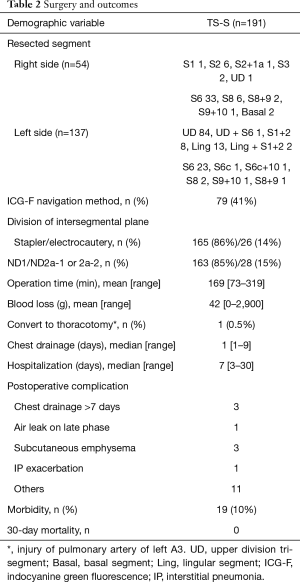
Surgery and outcomes are shown in Table 2. TS-Ss including sub-segmentectomies, were complete in all patients. To confirm the intersegmental border, ICG-F navigation was used in 79 patients (41%). We divided the lung parenchyma alone the intersegmental border by staplers in 165 patients (86%). In case with the tumor located near the intersegmental vein, we sometimes divided the lung by electrocautery to obtain the surgical margin. The surgical margin was negative for malignancy in all patients. Systemic nodal dissection (SND) was performed in 28 patients (15%). In case with pure GGN lesion, we usually omit SND. In some cases of compromised indication, we underwent SND (left upper division tri-segment 18, right S6 7, left S6 1, left lingular segment 1, right basal segment 1). Mean operative duration was 169 min (range, 73–319 min), and the mean blood loss was 42 mL (range, 0–2,900 mL). Unfortunately, one patient was converted to open thoracotomy (conversion rate 0.5%) because of the massive bleeding of pulmonary artery (A3) in left upper division segmentectomy (blood loss of this patient was 2,900 mL). Median duration of chest drainage was 1 day (range, 1–9 days), and the median postoperative hospital stay was 7 days, (range, 3–30 days). Postoperative complications occurred in 19 patients (10%). Prolonged air leaks persisting more than 7 days was in 3 patients, and subcutaneous emphysema in 3 patients. There was no in-hospital death and 30-day mortality, but one patient died of exacerbation of interstitial pneumonia on day 55 (90-day mortality 0.5%).
Full table
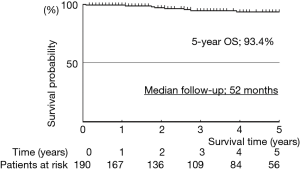
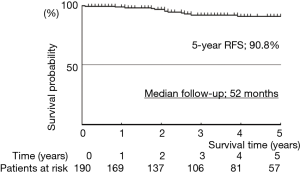
Median follow-up was 52 months. The actuarial survival rates of the 191 patients, including death from all cases, were 93.4% at 5 years (Figure 5). The relapse-free survival rates were 90.3% at 5 years (Figure 6). At the time of analysis, one patients had died of the disease. However, this case was synchronous bilateral multiple lung cancer, who was undergone left upper lobectomy by open thoracotomy for advanced side and then thoracoscopic right S6 segmentectomy was performed as a compromised indication. This case had brain and bone metastases from advanced site on 10 months after operation and died of disease on 69 months after operation. Eight patients died of other disease. Distal metastases occurred in 4 patients, including 1 brain and bone, and 3 pulmonary metastases in the other lobe. There was no local recurrence at the resected site.
Discussion
Lung segmentectomy started for the benign lung disease such as bronchiectasis and tuberculoma (3-5). However, recently, there were some reports of therapeutic effectiveness of segmentectomy for small-sized peripheral lung cancer (6-8). In addition, the indication of sub-lobar resection for peripheral GGN have been increasing due to recent advancements of CT screening. Wedge resection and segmentectomy are included in sub-lobar resection. Compared to wedge resections, segmentectomies have some advantages for the treatment of lung malignancy. One is that it is easy to obtain the sufficient surgical margin between the tumor and resected line. And the other is that it is possible to assess the hilar lymph nodes. From the technical aspect, segmentectomies are more complex procedure than lobectomies. We need to know anatomical variances preoperatively, expose and divide each pulmonary vessels and segmental bronchi during operation, and hilar lymph node dissections in segmentectomy are more difficult than that in lobectomy. Because segmental lymph nodes are often fragile and lobar lymph nodes are hide in the narrow spaces. We performed SND in 28 patients of compromised TS-S. Eighteen out of 28 (64%) patients were left upper division tri-segmentectomy. In this segmentectomy, we can dissect hilar nodes as same level as lobectomy. However, in left upper tri-segmentectomy, we should dissect the interlobar lymph node (station 11), because our data suggested that the interlobar lymph node involvement occurred in more than 20% cases (9). Even now, the indication of segmentectomy for radiologically invasive lung cancers has not been clear. A multicenter Japanese trial named “A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer” is recruiting patients with ≤2 cm peripheral radiologically invasive NSCLC (10). This study is a non-inferiority trial whose investigational arm is segmentectomy, the standard arm is lobectomy, and primary end-point is overall survival (OS). In this study, if hilar node is positive for malignancy, we need to convert to lobectomy because this study is non-inferiority trail. This study was now closed registration, so we are looking forward to having the results from this study whether we can indicate segmentectomies for invasive lung cancers.
Almost procedures and basic skills in lung segmentectomy are same as those in lobectomy. However, in segmentectomy, we need to dissect each vessels and bronchus more distally and confirm the intersegmental plane. There are two ways to make sure the intersegmental plane. One is using segmental bronchus and the other is using segmental artery. Okada et al. Reported the method of target-segment inflation by jet ventilation or needle insertion has been reported (11). However, this method is sometimes difficult to employ with a severely emphysematous lung and when visualization is limited, such as with VATS. Sekine et al. Reported the transbronchial injection of ICG and confirm the intersegmental plane with infrared thoracoscopy (12). A s a method of using segmental artery, Misaki et al. conducted a clinical trial of segmentectomy using an infrared thoracoscopy system with ICG (13). Each bronchus is associated with a pulmonary artery. They have succeeded in visualizing the differential blood flow of the pulmonary artery in the lung using infrared thoracoscopy with injection of ICG (3 mg/kg). Detailed macroscopic and microscopic examination confirmed that the marking corresponded to the intersegmental line. We have started this ICG fluorescence navigated TS-S method since 2013 and reported the initial results of this method in 2017 (14). Preoperative surgical-planning simulation evaluated by three-dimensional CT and intraoperative ICG fluorescence navigation is useful for anatomical segmentectomy during TS without inflation of the lung. After identification of the intersegmental plane, some surgeons like to divide the lung parenchyma with cautery cutting (11,15). In our institution, we usually use staplers to divide the lung parenchyma because of quickness and less complication after operation. In case with the tumor located near the inter segmental vein, we sometimes use a cautery cutting to obtain the surgical margin. Whether we use cautery cutting or stapler for parenchymal division is not main issue of TS-S. We should select to use them properly.
We have no postoperative local recurrences after TS-S. Despite a short follow-up period, our indication and results of TS-S was an acceptable. There are some reports of local recurrence after segmentectomy (16-18). And completion lobectomy after segmentectomy is technically more complicated procedure (19). Especially, early stage lung cancers such as GGN should be cure by surgical resection. Therefore, an initial operation is important. Lung segmentectomy has many variations, in addition, an operative indication of TS-S depends on surgeons’ decision. We should not depend on surgical skill only to do curative operation. Do not hesitate to do lobectomy for deeply located tumor.
Conclusions
Our indication and results of TS-S were acceptable. The most important issues of TS-S are patients’ selection and curability. We should wait for the results of a large scale RCT to indicate lung segmentectomy for the patients with radiologically invasive lung cancer
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Cancer Institute Hospital Institutional Review Board of Clinical Research, and the need for informed consent from patients was waived because of its retrospective design.
References
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg 1939;109:481-99. [Crossref] [PubMed]
- Blades B. Conservation of lung tissue by partial lobectomy. Ann Surg 1943;118:353-65. [Crossref] [PubMed]
- Overholt RH, Woods FM, Betts RH. An improved method of resection of pulmonary segments; report of a technique applied in 70 operations. J Thorac Surg 1948;17:464-79. [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [Crossref] [PubMed]
- Kuroda H, Sakao Y, Mun M, et al. Lymph Node Metastases and Prognosis in Left Upper Division Non-Small Cell Lung Cancers: The Impact of Interlobar Lymph Node Metastasis. PLoS One 2015;10. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Locoregional recurrence after segmentectomy for clinical-T1aN0M0 radiologically solid non-small-cell lung carcinoma. Eur J Cardiothorac Surg 2017;51:518-525. [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Omasa M, Date H, Takamochi K, et al. Completion lobectomy after radical segmentectomy for pulmonary malignancies. Asian Cardiovasc Thorac Ann 2016;24:450-4. [Crossref] [PubMed]