Lobectomy with angioplasty: which is the best technique for pulmonary artery reconstruction?
Introduction
Lobectomies with bronchial and/or vascular reconstruction are conservative procedures aimed at managing locally advanced lung cancer, avoiding a pneumonectomy. A conservative procedure with bronchial and/or vascular reconstruction, when feasible, is to be considered mandatory, provided that a radical resection can be achieved (1). Pulmonary angioplasty can be accomplished with different techniques according to the specific situation and the surgeon’s experience.
Despite the considerable evolution of these procedures, there is still no standard method and, the advantages and disadvantages of the available materials used for reconstruction are debated, as are those regarding several procedural steps (2).
This analysis is aimed at examining this topic, in particular the definitions, incidence, results, complications and spectrum of techniques available, in order to assess the acquired oncological and technical data.
Lobectomy with pulmonary artery (PA) reconstruction
The definition is unclear and not widely accepted. In literature, there is a large variety of inclusion criteria used to define a lobectomy with PA reconstruction. In some case series, even a simple tangential resection of the PA is considered to be a reconstructive/plastic procedure while, alternatively, some authors believe that the term PA angioplasty should be reserved only for arterial sleeve resection, reconstruction with conduits or for patch reconstructions (3). Somewhere between the first, overly-simplistic definition and the second overly-restrictive one, a PA reconstruction could be defined as “any kind of arterial wall resection, repaired by a hand suture requiring a proximal clamp, placed after systemic heparinization” (4,5). We favor the latter definition because it seems to be the most suitable in describing the principles of these procedures.
Incidence
Assessing incidence is impossible, firstly because there is not an accepted definition of these methods and secondly because there is a large variability in terms of frequency reported in published series. In any case, these procedures are performed uncommonly and roughly represent between a third and a quarter of all bronchial sleeve lobectomies. It has been estimated that, in thoracic surgery units devoted to conservative procedures, a PA reconstruction may be required in no more than 2% of all lobectomies (4,6).
Anatomy
Lobectomies with pulmonary angioplasty are more commonly performed on the left side, because of the anatomy of the left PA and, more specifically, owing to its relations with the left upper bronchus, and with the ipsilateral hilar and mediastinal nodal stations. The left PA is more frequently involved in two particular sites: (I) at the epibronchial level, proximally or distally to the first branch; and (II) at the intrafissural level, above the segmental artery for S6.
- At the epibronchial level, the left PA wall can be involved directly by the tumor or by N1 nodes (when they are placed distally to the first PA branch), or by N2 nodes (if the artery is infiltrated at the level of station #5 whose nodes are located proximally to the first branch) (7,8). The need for PA reconstruction, when the vessel is involved at this level, occurs during left upper lobectomies.
- At the intrafissural level, the left PA infiltration may involve the branches supplying the apicoposterior segment of the upper lobe or the segmental arteries for the lingula. A left upper lobectomy can be performed for PA involvement at this level with different reconstructive vascular procedures depending on the extent of the vascular infiltration. Sometimes, in centrally located cancers of the left lower lobe, the PA is involved at the level of the segmental artery for the S6. In such situations, an angioplasty is required in order to save the blood supply to the lingula, during a left lower lobectomy.
On the right side, the choice of conservative procedure of PA angioplasty is much less frequent. Sometimes the procedure is indicated for the involvement of the first branch of the right PA. The conservative procedure can be technically much more demanding than on the left side because the proximal clamp must be placed in a narrow space on the main right PA, below the superior vena cava.
Surgical oncology
A lobectomy with PA angioplasty is performed for locally advanced tumors which would otherwise necessitate a pneumonectomy to be removed. The oncological value is primarily related to the stage of disease and nodal status (9-12). Moreover, the tumor dimension, and the depth of the arterial wall infiltration have been associated with prognosis, in addition to the possible presence of neoplastic emboli (13).
Literature contributions regarding the value and feasibility of PA reconstruction in order to avoid a pneumonectomy first appeared in the late eighties and then increased in number and scientific impact (14,15). In the older literature, oncological results were reported to be worse than those associated with a standard lobectomy (16-18) but more recent articles show equivalent results to a standard lobectomy adjusted for stage (9,11,14,19-23), probably due to improvement in technique and experience with procedure. Concomitantly, there is a general agreement that conservative procedures lead to better results than pneumonectomies. Lausberg et al. report that PA reconstructions represent a middle point between bronchial sleeve resections and pneumonectomies when considering disease free survival and overall survival (24).
Complications
A lobectomy with PA reconstruction presents a number of possible complications such as lung infarction, bleeding, embolism, late PA stenosis, bronchovascular fistula and even cardiac dislocation at the site of pericardium harvesting (11,14,17,20,21,25-27). The incidence of these specific complications is quite variable in the different series, but since major or even lethal complications are possible, they must be carefully considered in preoperative surgical planning.
Most of these complications can be related to technical issues concerning the type of reconstruction and sometimes also the materials employed.
Techniques
The basic steps of a lobectomy with pulmonary angioplasty are as follows: (I) complete hilar dissection and mediastinal lymphadenectomy; (II) establish that the procedure is oncologically suitable; (III) harvest the biological graft if one of these options is chosen; (IV) achieve full control of the main and distal PA: proximally with a Satinsky clamp and distally with a tourniquet or by clamping the inferior (or the residual) pulmonary vein (PV); (V) administer heparin intravenously prior to the PA occlusion; (VI) incise the PA wall to create a defect with even margins; (VII) ascertain the presence of tumor-free margins by frozen section.
Direct reconstruction
The three methods available for direct PA reconstruction include: (I) tangential resection with direct suture; (II) transverse suture; and (III) arterial sleeve resection and end-to-end anastomosis.
Tangential vascular reconstruction with direct suture
A tangential resection is only performed in the case of limited PA wall infiltration, when a simple tangential mechanically-stapled suture is unfeasible. The ensuing longitudinal defect may be some centimetres long; a running suture with 5-0 or 6-0 non-absorbable monofilament material is placed along the longitudinal axis of the PA. Stenosis is the primary complication of this procedure (Figure 1).
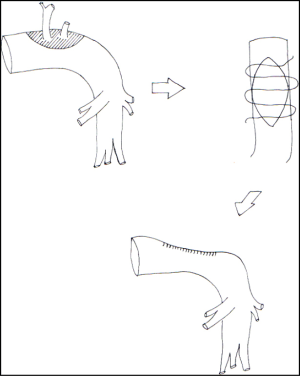
Transverse suture
This technique is feasible only in case of minimal resection of the PA wall on its longitudinal axis. The PA wall defect is sutured transversely, using a 5-0 or 6-0 monofilament non-absorbable running suture. Stenosis is thus avoided, but this kind of repair lead to a kinking of the PA which may be particularly severe in case of wide arterial wall defect or if a bronchial sleeve lobectomy has been added to the PA reconstruction. This method is not recommended and other reconstructive techniques should be considered (Figure 2).
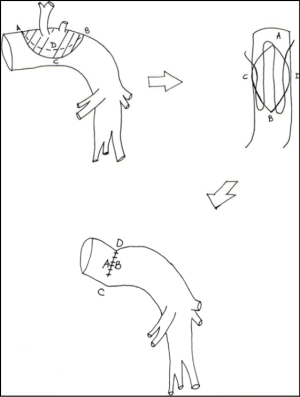
Circumferential resection and end-to-end anastomosis
A circumferential resection with an end-to-end anastomosis should be considered when the resection involves over 50% of the vessel’s cross-sectional area, thus precluding the use of a patch reconstruction. Most of arterial sleeve resections are performed along with a bronchial sleeve resection. In this setting, we prefer to complete the bronchial anastomosis before the vascular one for several reasons: (I) to reduce the manipulation of the vascular suture; (II) the post-reconstruction airway length is shorter and the tension on the vascular suture is therefore reduced; (III) the bronchial suture is theoretically easier to perform when the PA is divided. This kind of procedure should only be performed when strictly necessary (28) because entails a higher risk for specific complications (Figure 3). When both bronchial and vascular sleeve resections are performed, release maneuvers are generally unnecessary. As already mentioned before, the airway is shortened by the bronchial sleeve resection and the vascular stumps can be approximated without any tension. When only a vascular sleeve resection is performed, an extended sub-adventitial dissection of the PA is recommended to reduce the tension on the vascular anastomosis. Other “release” maneuvers can be performed, including an intrapericardial vascular dissection with a U-shaped incision around the inferior PV (29). In this way, the whole lung may be shifted cranially for a few centimetres, thus reducing the need to place a conduit to a very small number of cases.
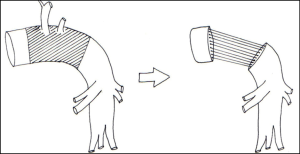
Graft reconstruction
Autologous tissues should be favored and the pericardium is the most used material even though heterologous grafts have been used (30). Azygous vein is theoretically suitable and favorable but is seldom used because most procedures are performed on the left side (28). Concerning the use of the saphenous vein, the impossibility of knowing with certainty before the thoracotomy, whether a PA will be performed or not, precludes the use of this option (31). The PV recently proved to be a very satisfactory method of reconstruction due to the tissue characteristics (28). Among heterologous biografts, the bovine and porcine pericardium can be effective but more understanding is needed because few reports are available (30,32). A synthetic graft (polytetrafluoroethylene) is generally considered to be avoided as there is too much difference in consistency with the thin and fragile wall of the PA (33).
As already mentioned, most procedures and most published series of patients describe the technique of graft reconstruction using the autologous pericardium. The main advantage of the pericardium is that it is readily available in large amounts but its disadvantages cannot be overlooked: it is non-stable, not vascular tissue and tends to curl (34,35). Moreover, a prosthetic repair of the residual pericardial defect is often required. More recently, the autologous PV has been proposed as a suitable graft for the PA repair. The graft is harvested by a preliminary sub-adventitial dissection of the vein, conducted from the intrapericardial tract to the peripheral main branches. The PV is then sutured with a linear stapler intrapericardially, as close as possible to the left atrium, and ligated peripherally. All the tissue included between the intrapericardial stump and the peripheral branches is harvested and dipped in 0.9% saline solution. Very often, it is possible to obtain a 2-cm long autologous vein graft to be utilized both as patch or conduit (36). In patch angioplasty, the harvested PV is opened longitudinally and tailored to cover the defect. The vein patch is secured by a running suture, conducted between two stay stitches. In vascular sleeve resections without a bronchial sleeve, a short PV graft may be interposed. The intrapericardial edge of the graft is anastomosed first to the proximal PA stump. A moderate caliber discrepancy generally occurs (Figure 4).
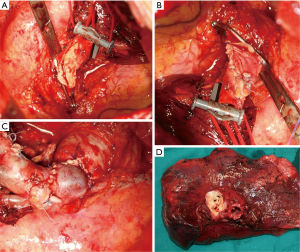
Discussion
Various techniques are available for PA reconstruction (37). Small residual defects after a tangential resection should be repaired by direct re-approximation of the vessel edges, provided that the resulting stenosis is <50%. A low-grade PA stenosis has no hemodynamic effects, particularly in light of the lower blood flow demand of the residual lung after the lobectomy. In our opinion, the “transverse” angioplasty is a procedure to avoid. Theoretically, the PA kinking induced by such enlarging angioplasty and worsened by the cranial shift of the remaining lobe may cause hemodynamic consequences, such as flow reduction and turbulence, which are worse than a moderate stenosis.
In moderately large defects, patch reconstruction is mandatory when direct repair would produce a stenosis greater than 50% and provided that the PA defect is repairable (28). Biological tissue is the preferred choice. The use of autologous or heterologous pericardium have been described for PA reconstruction (20,32). However, autologous pericardium seems not to be the ideal material for PA repair because it shrinks and curls with consequential technical problems and possible postoperative complications (28). To avoid such problems, the same authors have proposed stiffening the pericardium using a glutaraldehyde solution (34).
Although our experience with a PV patch is in its preliminary stage, we consider the PV, harvested from the lobe to be resected, an excellent source of autologous tissue for PA reconstruction. The physical properties of the PV are superlative; histologically, the PV wall is a unique vascular tissue with favourable thickness, resistance and a solid consistency. The wall of the PV is composed of a thin endothelium, a media of smooth muscle, and a thick outer fibrous adventitia (38). The smooth muscle of the venous wall overlaps the atrial myocardium and this myocardial sleeve may be found up to the main branches of the superior PVs. When a pulmonary angioplasty has been scheduled, the PV of the lobe to be resected should be harvested, in view of its possible use, provided that it is free of tumor. The PV always supplies sufficient tissue for a patch reconstruction by simply opening its wall longitudinally. The use of a PV graft is of course not indicated in case of direct involvement of the vein by the tumor. On the basis of an experience of over 20 cases, we have never observed neoplastic recurrences on the site of the angioplasty repaired with the autologous PV.
Very rarely, a conduit must be interposed between the arterial stumps after a complete sleeve resection. In our opinion, the PV graft shows significant advantages over the autologous pericardium conduit: the technique is simpler, quicker and safer, and the tissue is more suitable. The mild caliber discrepancy between the PA and the PV graft has no hemodynamic consequences, provided that the patient has a suitable anatomy. A 1.5- to 2-cm graft is adequate because excessive length of the conduit is undesirable, due to the risk of kinking, turbulent flow and possible thrombosis.
The main limitation of the use of the PV for pulmonary angioplasty is related to the possible unfavorable anatomy which can limit the availability of large graft tissue (common left PV, short PV due to an early division of the culminal and lingular branches) (39-41). Therefore, it is not always possible to use the PV graft for a conduit repair, but by a correct graft harvesting, it is always possible to obtain enough tissue for a patch repair.
Conclusions
In small PA defects, consider using longitudinal direct suture only when a simple stapled suture is unfeasible and when the residual stenosis is <50%. In larger defects, avoid any transverse angioplasty in favour of an autologous patch repair (the autologous PV grafting should be considered only provided that the PV is far from the site of the cancer). When the resection involves over 50% of the vessel’s cross-sectional area, carry out a complete sleeve resection with an end-to-end anastomosis. Furthermore, in exceptional cases when a prosthetic conduit is needed, use an autologous PV graft, when suitable. Finally, perform a pneumonectomy if the reconstructive final result is unsatisfactory.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Ibrahim M, Maurizi G, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. Thorac Surg Clin 2013;23:337-47. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Venuta F, et al. Bronchial and arterial resection for centrally-located lung cancers. J Thorac Dis 2016;8:S872-S881. [Crossref] [PubMed]
- Zhao LL, Zhou FY, Dai CY, et al. Prognostic analysis of the bronchoplastic and broncho-arterioplastic lobectomy of non-small cell lung cancers—10-year experiences of 161 patients. J Thorac Dis 2015;7:2288-99. [PubMed]
- Xu X, Huang J, Yin W, et al. Pulmonary arterioplasty using video-assisted thoracic surgery mechanical suture technique. J Thorac Dis 2016;8:617-27. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, et al. Thoracic and cardiovascular surgery in Japan during 2013. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- D’Andrilli A, Venuta F, Rendina EA. Pulmonary artery sleeve resection and lobectomy. In: Mathisen DJ, Morse C. editors. Master Techniques in Surgery: Thoracic Surgery: Lung resections, broncoplasty. Wolters Kluwer, 2014.
- Rendina EA, Venuta F. Reconstruction of the pulmonary artery. In: Patterson GA, Cooper JD, Deslauriers J, et al. editors. Pearson’s Thoracic & Esophageal Surgery. 3rd ed. Philadelphia, PA: Churchill Livingstone, Elservier, 2008:909-22.
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001. [Crossref] [PubMed]
- Venuta F, Ciccone AM, Anile M, et al. Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185-91. [Crossref] [PubMed]
- Yamashita M, Komori E, Sawada S, et al. Pulmonary angioplastic procedure for lung cancer surgery. Gen Thorac Cardiovasc Surg 2010;58:19-24. [Crossref] [PubMed]
- Alifano M, Cusumano G, Strano S, et al. Lobectomy with pulmonary artery resection: Morbidity, mortality, and long-term survival. J Thorac Cardiovasc Surg 2009;137:1400-5. [Crossref] [PubMed]
- Fadel E, Yildizeli B, Chapelier AR, et al. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg 2002;74:851-8; discussion 858-9. [Crossref] [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1153-61. [Crossref] [PubMed]
- Vogt-Moykopf I, Fritz T, Meyer G, et al. Bronchoplastic and angioplastic operation in bronchial carcinoma: long-term results of a retrospective analysis from 1973 to 1983. Int Surg 1986;71:211-20. [PubMed]
- Icard P, Regnard JF, Guibert L, et al. Survival and prognostic factors in patients undergoing parenchymal saving bronchoplastic operation for primary lung cancer: a series of 110 consecutive cases. Eur J Cardiothorac Surg 1999;15:426-32. [Crossref] [PubMed]
- Chunwei F, Weiji W, Xinguan Z, et al. Evaluations of bronchoplasty and pulmonary artery reconstruction for bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:209-13. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971-6; discussion 1976-7.
- Galetta D, Borri A, Gasparri R, et al. Surgical Techniques and Long-Term Results of Pulmonary Artery Reconstruction in Patients With Lung Cancer. Ann Thorac Surg 2015;100:1196-202; discussion 1202. [Crossref] [PubMed]
- Kojima F, Yamamoto K, Matsuoka K, et al. Factors affecting survival after lobectomy with pulmonary artery resection for primary lung cancer. Eur J Cardiothorac Surg 2011;40:e13-20. [Crossref] [PubMed]
- Xuegang L, Chao S, Zhen T, et al. Pulmonary artery reconstruction using autologous pericardium or azygos venae substitute for surgical treatment of central non-small cell lung cancer. Cell Biochem Biophys 2013;67:949-55. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lungcarcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Tscholl D, et al. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg 2005;79:1147-52; discussion 1147-52. [Crossref] [PubMed]
- Ayabe H, Kawahara K, Tagawa Y, et al. Results of pulmonary angioplastic operations for cases of bronchogenic carcinoma. Nihon Kyobu Shikkan Gakkai Zasshi 1990;28:278-83. [PubMed]
- Maggi G, Casadio C, Pischedda F, et al. Bronchoplastic and angioplastic techniques in the treatment of bronchogenic carcinoma. Ann Thorac Surg 1993;55:1501-7. [Crossref] [PubMed]
- Rendina EA, De Giacomo T, Venuta F, et al. Lung conservation techniques: bronchial sleeve resection and reconstruction of the pulmonary artery. Semin Surg Oncol 2000;18:165-72. [Crossref] [PubMed]
- Maurizi G, D’Andrilli A, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. J Thorac Dis 2016;8:S168-80. [PubMed]
- Ragusa M, Vannucci J, Cagini L, et al. Left main bronchus resection and reconstruction. A single Institution experience. J Cardiothorac Surg 2012;7:29. [Crossref] [PubMed]
- Galetta D, Veronesi G, Leo F, et al. Pulmonary artery reconstruction by a custom-made heterologous pericardial conduit in the treatment of lung cancer. Lung Cancer 2006;53:241-3. [Crossref] [PubMed]
- Yoshimi F, Amemiya R, Asato Y, et al. Pulmonary artery reconstruction using a great saphenous vein autograft in the treatment of bronchogenic cancer. Surg Today 1994;24:570-3. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Ciccone AM, et al. Long-segment pulmonary artery resection to avoid pneumonectomy: long-term results after prosthetic replacement. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [PubMed]
- Mei J, Pu Q, Zhu Y, et al. Reconstruction of the pulmonary trunk via cardiopulmonary bypass in extended resection of locally advanced lung malignancies. J Surg Oncol 2012;106:311-5. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Venuta F, et al. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005;80:357-8. [Crossref] [PubMed]
- Isenburg JC, Simionescu DT, Vyavahare NR. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials 2005;26:1237-45. [Crossref] [PubMed]
- Puma F, Capozzi R, Daddi N, et al. Experience with autologous pulmonary vein for pulmonary arterioplasty. Eur J Cardiothorac Surg 2011;40:e107-11. [PubMed]
- Venuta F, Ciccone AM. Reconstruction of the pulmonary artery. Semin Thorac Cardiovasc Surg 2006;18:104-8. [Crossref] [PubMed]
- Ho SY, Cabrera JA, Tran VH, et al. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 2001;86:265-70. [Crossref] [PubMed]
- Cerezo F, Cano JR, Espinosa D, et al. New technique for pulmonary artery reconstruction. Eur J Cardiothorac Surg 2009;36:422-3. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Pulmonary artery reconstruction with pulmonary vein conduit for lung cancer: medium-term results. Ann Thorac Surg 2014;98:990-5. [Crossref] [PubMed]
- Hirai A, Shinohara S, Kuwata T, et al. Pulmonary artery reconstruction using autologous pulmonary vein for surgical treatment of locally advanced lung cancer: a case report. Surg Case Rep 2016;2:46. [Crossref] [PubMed]


