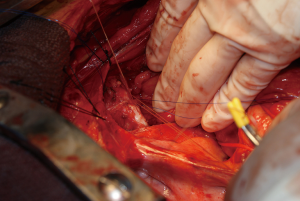
Combined esophagectomy and carinal pneumonectomy
Introduction
The trachea and esophagus share a common blood supply from a longitudinal artery along the tracheoesophageal groove on each side. Esophagectomy, especially with lymph node dissection, compromises the blood supply to the trachea and carina resulting in ischemia at the anastomosis and predisposing to failure. As a result, combined esophagectomy with circumferential tracheal or carinal resection and reconstruction has been reported only sporadically (1-4).
Carinal pneumonectomy is considered one of the most challenging airway surgeries by itself. Successful combined esophagectomy and carinal pneumonectomy has never been reported before. In the following case report, the situation arose that combined esophagectomy and left carinal pneumonectomy was the only choice to provide the patient with meaningful palliation and the patient gained ten more months of useful time to spend with his family.
Case presentation
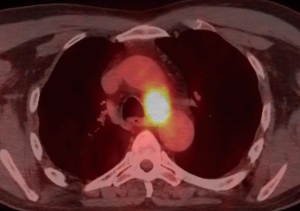
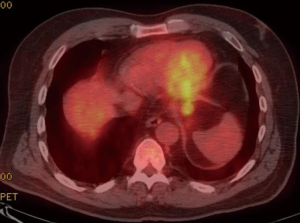
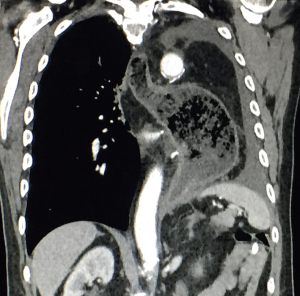
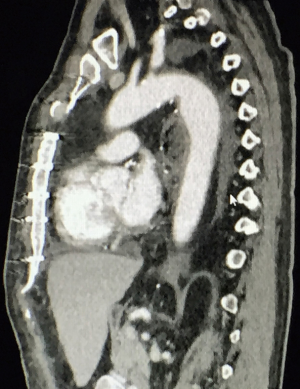
A 59-year-old man experienced hoarseness of voice and was found to have left recurrent nerve paralysis. CT chest demonstrated a poorly marginated soft tissue mass that appeared to originate from the anterior surface of the mid-esophagus measuring 2.6 cm ×3.7 cm and extended to the aortopulmonary window encroaching on to the aortic arch. PET scan showed asymmetric thickening in the middle one-third of the esophagus with increased FDG activity (SUVmax of 9.0), highly concerning for primary esophageal malignancy (Figure 1). The enlarged aortopulmonary window mass or lymph node was also FGD avid (Figure 2). There was an enlarged FDG avid lymph node at the gastroesophageal junction in the upper abdomen (Figure 3). EGD showed no esophageal mucosal lesion but needle aspiration of the aortopulmonary window mass showed squamous cell carcinoma. The clinical diagnosis was esophageal squamous cell cancer with lymph node metastases involving the aortic arch and left recurrent nerve and lymph node at the gastroesophageal junction. There was no evidence of systemic metastasis.
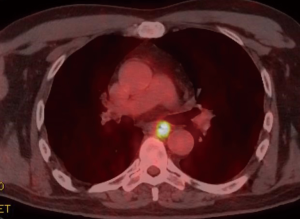
He was deemed not a surgical candidate and was treated with chemoradiation with carboplatin and paclitaxel weekly given during radiation therapy (60 Gy delivered to the PET defined abnormalities). PET scan after completion of treatment showed no improvement and repeat EGD now showed an esophageal ulcer at 32 cm and biopsy showed squamous cell cancer as well. Two months later, CT chest showed the aortopulmonary window mass was enlarging and compressing the trachea and left main bronchus. The patient started to develop hemoptysis.
Facing non-responsiveness of chemoradiation and newly developed airway invasion with hemoptysis, impending development of esophagobronchial fistula and miserable death, the patient requested re-evaluation for surgical resection despite being informed that surgical resection would be technically challenging, possibly not feasible, would not be curative, and has a high chance of morbidity and mortality. Complete resection would require combined esophagectomy and left carinal pneumonectomy with possible resection of the aortic arch. Discussion with patient and his wife was performed on several occasions to make sure they understood the situation and a detailed informed consent was obtained.
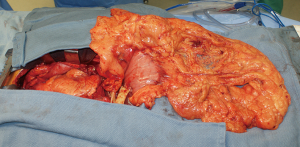
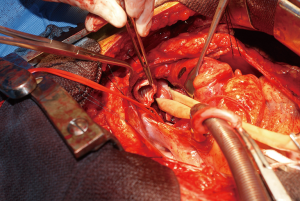
During surgery on August 7, 2015, he was ventilated with a single lumen endotracheal tube. Bronchoscopy confirmed tumor invasion of the left lower trachea and left main bronchus. He was explored through an incision from the suprasternal notch to the umbilicus. After preliminary mediastinal exploration showing promise that resection could possibly be performed, the stomach was mobilized together with the entire omentum (Figure 4). A jejunostomy was placed. He was systemically heparinized and then put on normothermic cardiopulmonary bypass by cannulating the right atrium and left common femoral artery. Since there was cancer and potential bacterial contamination due to subsequent opening of the airway and gastrointestinal tract, we did not use cell saver or pump sucker. We transected the trachea above the level of cancer involvement (Figure 5), the right main bronchus, the superior mediastinal esophagus, the left main pulmonary artery and the left pulmonary veins. We were pleased to find that the tumor was completely detachable from the media of the aorta and aortic arch resection was not needed. The resected en-bloc specimen consisted of the left lung, left main bronchus, distal trachea with carina, esophagus with proximal stomach and the gastroesophageal junction lymph node and tumor below the aortic arch (Figure 6).
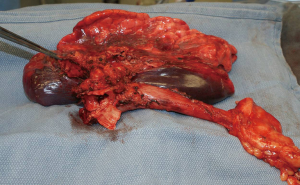
The stomach was brought to the posterior mediastinum and a stapled anastomosis with the superior mediastinal esophagus using a 25 mm EEA stapler was performed. The right main bronchus was anastomosed to the trachea using 4-0 polypropylene (continuous for the posterior membranous wall and interrupted figure of eight for anterior cartilaginous wall) (Figure 7). The entire greater omentum attached to the stomach was brought to the chest and used to wrap the airway anastomosis and fill up the left pleural space to prevent post-pneumonectomy empyema. The patient was then weaned off cardiopulmonary bypass and the incision was closed. Cardiopulmonary bypass time was 5 hours and the entire procedure took 10 hours.
He was extubated 1.5 days after surgery. He developed sputum retention requiring a mini-tracheostomy and repeated bronchoscopy. He first ambulated 5 days after surgery and he walked home on room air 2 weeks after surgery. CT scan 1 month after surgery showed patent and intact trachea to right main bronchus anastomosis nicely wrapped by omentum (Figure 8), a healthy intrathoracic stomach (Figure 9), and absence of tumor around the aortic arch (Figures 8,10).

Subsequently, he did develop necrosis at the airway anastomosis and there was a small anterior fistula into the mediastinum. It did not cause real problem because it was covered by omentum and it spontaneously drained back into the trachea. Later on, he developed tracheal stenosis but it was well treated by placement of a 14 mm × 20 mm covered stent (Merit Medical Systems, Inc., South Jordan, UT 84095, USA).
He was eating well and there was no more hemoptysis. He had no trouble breathing and spent quality happy time at home with his family. Unfortunately, he developed diffuse metastases 9 months after surgery and expired 10 months after surgery.
Discussion
Thompson reported the first successful combined esophagectomy and lower tracheal and carinal resection with reconstruction in 1973 (1). A 34-year-old man in Rhodesia had a squamous cell cancer of the esophagus that had invaded the membranous wall of the trachea. He first performed a left thoracotomy to assess the operability and to mobilize the stomach. Then, he performed a right thoracotomy with esophagectomy with resection of 10 rings of the lower trachea, carina and bilateral proximal main bronchi. The two residual main bronchi were reunited to form a new carina to be re-anastomosed to the remaining trachea. The patient was then turned supine and through a cervical collar incision, the esophagus was anastomosed to the gastric tube. The only complication was left vocal cord paralysis and a weak right vocal cord. He was able to eat solid food. Bronchoscopy two weeks later showed the new carina was healing well. The patient was presumably discharged back to his village and there was no report of long term follow up.
Subsequent reports were mainly from Japan. Ikeda et al. reported three cases in 1984 (2). They survived 8 days, 5 and 10 months. Kawahara in 1992 reviewed 18 Japanese patients with esophageal cancer undergoing concomitant sleeve resection of the tracheobronchial tree. Eight died within 1 month and the others died within a year except for one who died at 18 months (3). Matsubara et al. in 1995 reported their 20-year experience in patients who had esophageal cancer involving the major airway (4). The hospital mortality rate in the 55 esophagectomy cases was 30% in the first 27 cases (first 22 years) and 7% in the last 28 cases (last 8 years). The outcome in patients who underwent curative resection was significantly more favorable (P<0.0001), and the 2-year survival was 51%. The patients with non-resectable cancer all died within 6 months compared with a 23% 1-year survival rate for palliative esophagectomy cases (P<0.006).
Although combined esophagectomy and pneumonectomy has been reported (5), the authors are not aware of any prior report of combined esophagectomy and carinal pneumonectomy.
As pointed out by Grillo (6) and Salassa (7), the trachea and the upper esophagus share blood supply from a longitudinal artery along each tracheoesophageal groove. Performing esophagectomy would damage the blood supply to the trachea resulting in ischemia at the anastomosis after simultaneous tracheal resection. As a result, combined esophagectomy and tracheal resection has been regarded as extremely high risk due to high likelihood of complication at the tracheal anastomosis. This is the main reason why combined esophagectomy and tracheal resection and reconstruction has infrequently been attempted. This case confirms that combined esophagectomy and tracheal resection can result in airway ischemia and anastomotic complication.
The omentum is well known to be the surgeon’s friend especially in challenging situations encountered by the cardiothoracic surgeon (8). It provides a fresh blood supply to ischemic tissues to help them heal. It walls off leak and contains infection. It is soft and pliable so that it can conform to any irregular space. It provides a bulky healthy tissue to prevent postpneumonectomy empyema. It was well illustrated in this patient that the occurrence of a tracheal mediastinal fistula was made benign due to the presence of the omentum outside.
Our young unfortunate patient had a locally aggressive esophageal squamous cell cancer that had invaded the aortopulmonary window not responding to chemoradiation and subsequently invaded the trachea and left main bronchus resulting in hemoptysis, with impending bronchoesophageal fistula and a miserable death. He was advised repeatedly that traditionally, surgery would not be offered in his situation and the anticipated surgery had never been reported before. It was with his complete understanding of the severe magnitude and potentially fatal complications that the procedure was undertaken.
Since the pathology was in the center of the chest involving several vital structures: airway (trachea, carina, left main bronchus), esophagus, aortic arch and the heart, a reliable strategy to support the respiratory and circulatory systems was needed. The use of cardiopulmonary bypass was invaluable in performing such extensive operations (9). This allowed decompression of the heart improving exposure. Traction and displacement of the heart for the extensive surgery was also tolerated. With the patient on cardiopulmonary bypass, one could transect the airway and take the time to perform the best airway anastomosis without requiring the presence of an endotracheal or endobronchial tube. We did not employ pump sucker or cell saver to eliminate the chance of disseminating cancer or infection.
Conclusions
The first successful combined esophagectomy and left carinal pneumonectomy is being reported. Combined esophagectomy and airway resection can result in airway anastomotic ischemia and predisposes to dehiscence but the morbidity can be minimized by wrapping the anastomosis with omentum.
Acknowledgements
The authors are indebted to the late father of modern-day tracheal surgery, Hermes C. Grillo, MD [1923–2006], whose enormous contribution to airway surgery relieved the suffering of many patients throughout the world. The authors were able to complete this work due to the foundation laid down in airway surgery by Dr. Grillo. The authors admire the tremendous courage exhibited by the patient who sincerely and fearlessly requested to undergo this ultra-high-risk procedure.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient has passed away. His wife consented to the publication.
References
- Thompson DT. Lower tracheal and carinal resection associated with subtotal oesophagectomy for carcinoma of oesophagus involving trachea. Thorax 1973;28:257-60. [Crossref] [PubMed]
- Ikeda T, Sakai T, Sakai S, et al. Resection of the carina and oesophagus for malignant tumours of the oesophagus or tracheobronchial tree. Thorax 1984;39:201-5. [Crossref] [PubMed]
- Kawahara H, Shiraishi T, Yoshida Y, et al. Surgical treatment for esophageal cancer invading the adjacent organs. Shujutsu (Operation) 1992;46:865-978.
- Matsubara T, Ueda M, Nakajima T, et al. Can esophagectomy cure cancer of the thoracic esophagus involving the major airways? Ann Thorac Surg 1995;59:173-7. [Crossref] [PubMed]
- Tachimori Y, Watanabe H, Kato H. Left Pneumonectomy Associated with Subtotal Esophagectomy for Carcinoma of the Esophagus Invading the Left Main Bronchus. Jpn J Clin Oncol 1989;19:167-9. [PubMed]
- Grillo HC. Tracheal blood supply. Ann Thorac Surg 1977;24:99. [Crossref] [PubMed]
- Salassa JR, Pearson BW, Payne WS. Gross and microscopic blood supply of the trachea. Ann Thorac Surg 1977;24:100-7. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC, Vlahakes GJ, et al. The omentum in the management of complicated cardiothoracic problems. J Thorac Cardiovasc Surg 1988;95:677-84. [PubMed]
- Wiebe K. Extended pulmonary resections of advanced thoracic malignancies with support of cardiopulmonary bypass. Eur J Cardiothorac Surg 2006;29:571-7; discussion 577-8. [Crossref] [PubMed]