Prognostic factors of oligometastatic non-small cell lung cancer: a meta-analysis
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer mortality in man and women worldwide (1). Approximately half of all patients diagnosed with NSCLC, at the same time, present metastatic diseases. Despite the improved surgical techniques and advanced therapeutic regimens, its 5-year overall survival (OS) is still only about 18% (2). Unfortunately, more than 70% of cases are diagnosed at an advanced stage of the disease, and loss optimal opportunity for therapies (1).
The inspiration for this review depends on the strength of the rationale about cancer spread proposed by Hellman and Weichselbaum. Among the spectrum of locally identified to widely metastatic cancer, there exists an intermediate “oligometastatic state” where metastases are limited in number and location (3). It had hypothesized that in selected oligometastatic patients, locally aggressive therapies given with the intent of eradicating all sites of known metastatic sites could result in long-term survival, or even cure (4). Though multiple retrospective case series have reported long-term survival in oligometastatic NSCLC patients treated with curative intent (5), however, some scholars have suggested that the long-term survival outcomes observed in such studies may be more reflective for patient’s selection, rather than treatment effect (5,6). Thus, there is a great need for clearly defined parameters to identify which patients might have a benefit survival of oligometastatic NSCLC.
A prognostic factor explaining part of the population heterogeneity is a variable measured in individual patients and is at the time of diagnosis able to provide information on clinical guide and select patient (7). Some independent prognostic factors have been identified in order to predict survival and to help in the management of patients with resectable NSCLC (8), such as performance status, age, TNM stage, and TNM stage, age, sex, weight loss with advanced NSCLC (9). Since several emerging reports in the articles describing the prognostic factors of NSCLC, and the available studies permitting the delineation of factors predictive of long-term survival of oligometastatic NSCLC were rare. Therefore, we performed a meta-analysis to identify more benefit factors for long-term OS of oligometastatic NSCLC.
Methods
Search strategy and selection criteria
In cooperation with a trained librarian, we identified articles by searching PubMed, EMBASE and the Cochrane from inception to Mar 1, 2017 to ensure that all possible studies were found. The search strategy included the following terms combined of “non-small cell lung cancer OR non-small cell lung carcinoma OR non-small cell lung neoplasm OR non-small cell lung tumor OR NSCLC OR pulmonary adenocarcinoma OR lung adenocarcinoma OR adenocarcinoma of the lung OR lung squamous carcinoma OR pulmonary squamous carcinoma OR squamous cell lung carcinoma” AND “metast* OR oligomet* OR stage IV OR late stage OR advanced stage” AND “risk* OR prognos* OR epidemiology OR etilogy”. We restricted our searches to reports published in English.
Two reviewers independently screened the titles and abstracts of retrieved articles. This systematic review incorporated studies that reported populations meeting the following inclusion criteria: (I) NSCLC confirmed histologically; (II) NSCLC patients with 1–5 synchronous or metachronous metastases. (A synchronous metastasis was defined as being diagnosed at the same time or within 2 months of the primary lung and metachronous as ≥2 months after the histological diagnosis of primary lung); (III) a controlled primary tumor (defined as previous or current treatment of the primary tumor with radiation, primary surgery, or combination, with or without systematic chemotherapy); (IV) a uncontrolled primary tumor (defined as palliative treatment or without any primary treatment); (V) reported outcome of interest (i.e., OS); (VI) from an original study (i.e., retrospective study). Articles were excluded based on the following criteria: (I) not oligometastatic NSCLC; (II) duplicate articles; (III) the outcomes of interest [OS, hazard ratios (HRs)] of oligometastatic NSCLC patients could not be ascertained or separately analyzed; and (IV) data was insufficiently provided or was not extractable. The full text article of any study that appeared to meet the inclusion criteria was retrieved for further examination. Disagreements between reviewers regarding data abstraction were resolved through discussion.
Data extraction and study quality
For the review, the same reviewers extracted the data independently using standard data collection forms. The following information retrieved from all original reports: primary author, year of publication, number of eligible patients, age, gender, type of study, follow-up time, (y)pT and N-stage of the primary lung cancer (AJCC 7th edition), histology of primary lung cancer, thoracic stage [Union for International Cancer Control (UICC) 7th edition], treatment of primary lung cancer or both sites (primary and metastatic sites), OS and prognostic factors (univariate and multivariate). Preferred index for evaluating the risk of prognostic factors was HR. The primary endpoint of interest was OS which was defined as time to death from any cause, or to end of follow-up (censored).
The quality of eligible articles was evaluated by a modification of the Newcastle-Ottawa Scale (NOS). Full-length articles were all available for review. Among the included studies, matching criteria were variable, and little matching information was identified from the conference abstracts.
Statistical analysis
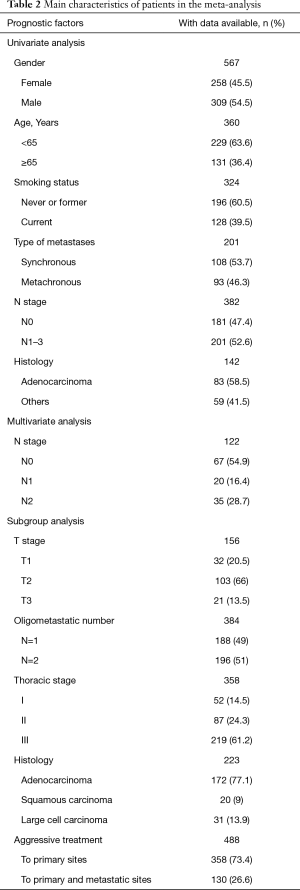
We analyzed data from all included patients, as well as the following baseline characteristics: sex, age (<65 vs. ≥65 years), smoking status (never or former vs. current), (y)pN stage (N0 vs. N1–3), tumorous histology (adenocarcinoma vs. others).
We did further exploratory analyses in the subgroups for the following factors: (y)pTNM stage [(y)pT1 vs. (y)pT2 vs. (y)pT3, (y)pN0 vs. (y)pN1 vs. (y)pN2], thoracic stage (I vs. II vs. III), tumorous histology (adenocarcinoma vs. squamous carcinoma vs. large cell carcinoma), oligometastatic number (n=1 vs. n=2 vs. n=3–5), aggressive therapy (AT) (AT to primary lung site vs. AT vs. AT to both sites), in order to describe possible heterogeneity of prognosis.
We calculated HR and 95% CIs for OS with Cox proportional hazards regression. We did an integration test of prognostic factors efficacy for each subgroup for all outcome index. We also analyzed the primary endpoint by trail, with all patients who were originally included in the eligible articles. These HRs and CIs slightly differ from the original articles whose definite follow-up information was not provided.
We calculated I2 and Q to assess whether significant heterogeneity existed between the included studies and did statistical analyses with Review Manager (version 5.3.0). P value of 0.05 or less was considered as statistically significant.
Results
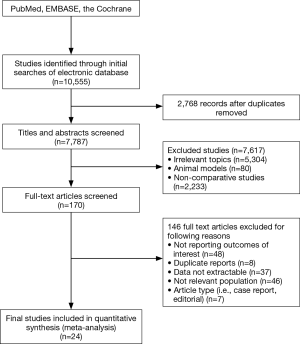
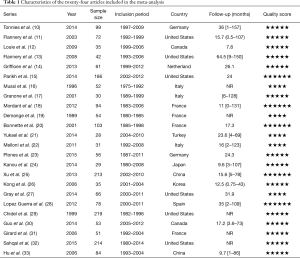
Our initial search identified 7,787 potentially eligible articles and excluded 7,617 citations by title that did not meet eligibility criteria. Finally, we ensured 24 eligible publications according to study the abstracts of the remaining 170 articles (Figure 1). Among these eligible articles, 22 full-texts (10-33) were retrospective trials and two articles were conference abstract. One abstract was presented during the European Society for Radiotherapy and Oncology in 2014, while anther was presented in 2015. We were able to extract prognostic factors for each article. Table 1 shows the main characteristics of included studies.
Full table
Included trials were published between 1989 and 2014. The selected trials were conducted in United States [6], France [4], Italy [3], Germany [2], Canada [2], China [2], Japan [1], Turkey [1], Spain [1], Korea [1], and Netherland [1]. Of the twenty-four articles, there were four literatures reporting synchronous and metachronous metastasis, twenty studies exclusively involving synchronous metastasis. A total of 1,935 eligible participants were included and the sample size ranged from 28 to 219 patients. Table 2 describes the main characteristics of the selective patients in the meta-analysis.
Full table
Univariate analysis
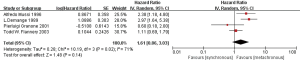
Firstly, we compared the effects on the prognosis of oligometastatic NSCLC between synchronous and metachronous metastasis. According to the quantitative synthesis, we detected no significant differences in OS (HR 1.61, 95% CI: 0.86–3.03; P=0.14) (Figure 2).
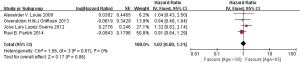
Then, we further explored prognostic factors of synchronous oligometastasis in NSCLC patients. Among these patients, the results showed that factors have no prognostic value among patients between younger and older than 65 years old (HR 1.02, 95% CI: 0.80–1.31; P=0.86, I2=0%) (Figure S1), as well as smoking status (HR 1.08, 95% CI: 0.80–1.46; P=0.62, I2=0%) (Figure S2). Female had slightly better prognosis (HR 1.21, 95% CI: 1.02–1.45; P=0.03, I2=22%) (Figure 3A). (y)pN0 existence might be significant benefit prognosis compared with (y)pN + (N1–3) (HR 1.82, 95% CI: 1.40–2.36; P<0.00001, I2=19%) (Figure 3B). Similarly, the result of tumorous histology demonstrated adenocarcinoma was favorite prognostic factor in the cohort (HR 1.44, 95% CI: 1.10–1.88; P=0.008, I2=49%) (Figure 3C).

Multivariate analysis
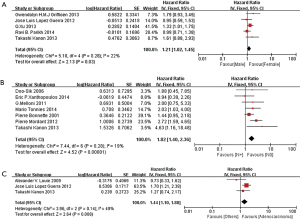
In multivariable analysis, we also extracted data from 2 studies including 122 synchronous metastases in NSCLC patients, and found a survival benefit with (y)pN0 stage compared with (y)pN1 stage (HR 1.63, 95% CI: 1.27–2.10; P=0.001, I2=0%), but no significant difference between (y)pN0 stage and (y)pN2 stage (HR 2.01, 95% CI: 0.80–5.03; P=0.14, I2=52%) (Figure S3).
Subgroup analysis
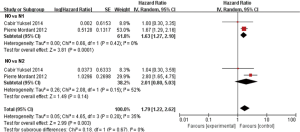
In subgroup analyses among prognostic factors, we recorded significant differences in OS of prognostic factors including tumorous histology, oligometastatic number, (y)pT-stage, Thoracic stage of synchronous oligometastasis from NSCLC (Figures 4,S4). And, patients with synchronous metastasis from NSCLC had a survival benefit with thoracic stage T1 compared with stage T3 (HR 2.07, 95% CI: 1.14–3.76; P=0.02) (Figure S5). Similarly, Figure 4A shows that patients diagnosed with thoracic stage I had a better prognosis in OS in contrast with either stage II (HR 1.69, 95% CI: 1.06–2.69; P=0.03) or stage III (HR 2.39, 95% CI: 1.52–3.75; P=0.0002). In contrast with patients whose progress was stage III of synchronous metastasis in NSCLC, patients diagnosed stage I or II had a positive prognosis in OS (HR 2.33, 95% CI: 1.01–5.38; P=0.05). In this review, we detected obvious benefit of patients with adenocarcinoma diagnosed pathologically in OS compared with squamous carcinoma or large cell carcinoma (HR 2.89, 95% CI: 1.41–5.92; P=0.004; HR 3.03, 95% CI: 2.01–4.57; P<0.00001, respectively) (Figure 4B). We also found that patients had a significant survival benefit with AT to primary sites, but no significant difference with AT to primary and metastatic sites (HR 0.43, 95% CI: 0.32–0.57; P<0.00001; HR 1.12, 95% CI: 0.67–1.88; P=0.67, respectively) (Figure 5). Furthermore, we observed that neither aggressive thoracic treatment [ATT, defined as thoracic surgery ± chemoradiotherapy (CRT)] (HR 0.43, 95% CI: 0.32–0.57; P<0.00001), nor ATT compared with CRT alone (HR 0.4, 95% CI: 0.16–1.01; P=0.05) were significantly associated with improved survival. But interestingly, upfront addition of radiotherapy (RT) to aggressive treatment (AT) to primary sites shows its negative prognosis in OS (HR 1.89, 95% CI: 1.08–3.31; P=0.03) (Figure 6). We found no heterogeneity among positive prognostic factors including (y)pT stage of primary sites, tumorous histology, thoracic stage, ATT.
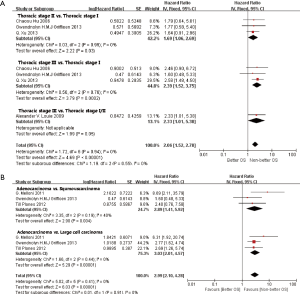
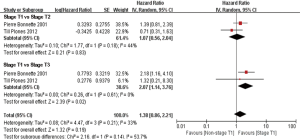

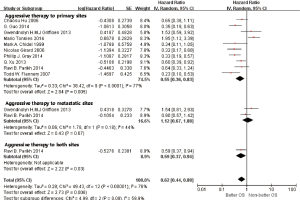
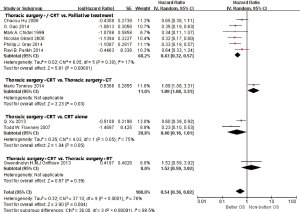
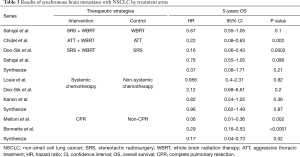
Table 3 shows the exploratory analysis in OS of synchronous brain metastasis from NSCLC by treatment arms. The pooled HR with Gamma knife radiosurgery (GKRS) plus whole brain radiation therapy (WBRT) was 0.37; systemic chemotherapy was 0.96; complete lung resection was 0.17. Because of limited analysis of retrospective studies, the result showed no significant difference in treatment arms of GKRS plus WBRT compared with WBRT alone (P=0.21), but an obvious survival benefit between WBRT plus ATT and ATT alone (HR 0.22, P=0.002) in OS.
Full table
Discussion
Since Martini and Melamed (34) firstly published a literature to clarify the definition of multiple primary NSCLC and solitary metastases, and later Hellmann and Weichselbaum (3) described the term “oligometastases” as a restricted loco-regional tumor load in 1995. Based on the UICC 8th edition, the stage of M1b was correspondence with concept of oligometastases. And despite the treatment for isolated distant metastases is gaining more and more momentum in the oncologic literature, some scholars found that the long-term survival outcomes observed might be more reflective for patient selection, rather than treatment effect (6). Moreover, the available studies assessing factors for long-term survival of oligometastatic NSCLC were rare. Thus, performing a meta-analysis on prognosis of oligometastases in NSCLC shows its great need.
Firstly, our finding shows that no significant benefit of metachronous metastases compared with synchronous metastases (HR 1.61, P=0.14). Several studies have evaluated the prognostic significance of synchronous versus metachronous metastases, and although none have reported significance in multivariable analyses, some previous studies’ experiences suggested that the synchronous metastases presentation of lung cancer results in a negative prognostic factor (18,35,36). According to this meta-analysis, overall, the result that synchronous metastases presented as a negative prognostic factor is basically consistent with previous reports.
NSCLC is a heterogeneous disease (37). Patients in NSCLC with advanced stage are also full of heterogeneity. Synchronous solitary metastasis is assumed to be stage IV, as is widely distributed metastatic disease. Furthermore, patients categorized as stage IV with symptoms or not, usually receive systemic treatments (18). Although these systemic therapies are appropriate for the majority of patients with stage IV NSCLC, patients with synchronous solitary metastasis with positive prognosis may get more benefit from this strategy than those with negative prognosis. For these patients, more aggressive local therapies may be advantageous (38,39). However, to our knowledge, there are relatively few reports concerning prognostic factors for patients with primary NSCLC and synchronous solitary metastasis, which could distinguish the distinct subset as a group that requires more ATs.
Therefore, we further comprehensively explored the prognostic factors of synchronous oligometastases NSCLC patients. On univariate analyses, we found that neither age (P=0.86, I2=0%), nor smoking status (P=0.62, I2=0%) had significant difference in OS. However, prognoses such as female (P=0.03, I2=22%), absence of nodal disease (P<0.00001, I2=19%), adenocarcinoma histology (P=0.008, I2=49%) were clarified as positive factors. In subgroup analyses, comparisons among the studies were limited by small sample sizes, heterogeneity in patient populations, and types of treatments delivered. Moreover, the primary tumor N-stage was found to be a significant prognostic factor for survival in multivariable analyses, and we further reflected the absence of nodal disease could achieve a survival benefit in oligometastatic NSCLC. By examining survival results across studies, this review also suggested that the thoracic T stage of synchronous oligometastases in NSCLC might also be prognosis for survival. However, survival outcomes in studies that included patients with T1 stage diagnosed pathologically might have a better survival benefit than those of patients with T3 stage (P=0.02, I2=0%), but no significant difference compared with patients with T2 stage (P=0.83, I2=44%). Interestingly, low number of metastatic lesions, which had been associated with favorable progression-free survival (PFS) in other case series (39-41), but were not significantly associated with longer OS in our cohort. The discrepancy between the findings of other studies and ours may be explained by the cause that previous trials included only patients with definitive treatment to metastases, whereas our study screened all NSCLC patients with oligometastatic disease, regardless of treatment received.
Subsequent to these findings, several prognostic factors influencing survival in patients suffering from NSCLC with synchronous metastases have been described in the medical literature (16,42,43), and ATT in the form of lung resection, as well as absence of nodal disease (44), adenocarcinoma histology, and stage I/II disease have commonly been described as playing positive role in survival. However, the prognosis of ATT and various treatment strategies for synchronous metastases in NSCLC remains unclear. Therefore, in the cohort, we further assessed the benefit of aggressive therapies, and investigated the role of different ATs for synchronous metastases in NSCLC. Our finding suggested that neither ATT (HR 0.43, P<0.00001) nor ATT compared with CRT alone (HR 0.4, P=0.05) were significantly associated with improved survival. But observingly, upfront addition of RT to AT showed its negative prognosis (HR 1.89, P=0.03), and chemotherapy to AT had no significant difference in OS (P=0.39). However, owning to the limitation of retrospective studies and the number of articles included, more prospective studies are need to be further verified.
In the review, approximately two-third of the patients was diagnosed as synchronous metastases, especially synchronous brain metastases (SBM). Thus, we further investigated risk of variously therapeutic methods among the SBM. Despite the integrated results showed no significant difference, we fund differently combinative strategies presented a better survival than one treatment received separately.
In the meantime, several limitations to this review should be cautiously observed. Firstly, a retrospective analysis cannot replace randomized controlled trials (RCTs). Thus, these results should be interpreted with caution. Secondly, it is possible that patients receiving definitive local therapy to the primary tumor were more likely to have additional favorable characteristics which we could not control, and due to the different time of publication of included literatures, the data on RT use and in general on therapeutic approach are surely biased by baseline different characteristics. Thirdly, we were not able to assess prognosis in PFS, local recurrence (LC) and the toxicity of treatment because of relatively insufficient data. Finally, more than two-third of metastatic sites involved in the cohort was synchronous brain metastasis. Therefore, establishing the benefit in other oligometastatic location in NSCLC is extremely necessary. However, except for the absence of randomized trials, retrospective analysis can play a positive role in clinical guild and provide the basis for future randomized comparisons.
Conclusions
Among patients with oligometastatic disease, defined as 5 or fewer lesions, we identified several factors associated with improved survival and they were consistently found to be significant in the literature. Key determinants of long-term survival of oligometastases in NSCLC patients include female, lower nodal stage, adenocarcinoma histology, thoracic stage, as well as AT to the primary tumor. We propose that these factors be used in future prognostic models to identify those oligometastatic NSCLC patients who are most likely to be long-term survivors, and be utilized to guide clinical selection making and the design of future prospective randomized studies.
Acknowledgements
Funding: This study was funded by National Natural Science Foundation of China (81572279), University Excellent Young Teachers Program of Guangdong Province (Yq2013040), Natural Science Foundation of Guangdong Province (2015A030313253), and Pearl River Nova Program of Guangzhou City (2014J2200031).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Macdermed DM, Weichselbaum RR, Salama JK. A rationale for the targeted treatment of oligometastases with radiotherapy. J Surg Oncol 2008;98:202-6. [Crossref] [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [Crossref] [PubMed]
- Utley M, Treasure T. Interpreting data from surgical follow-up studies: the role of modeling. J Thorac Oncol 2010;5:S200-2. [Crossref] [PubMed]
- Yip D, Harper PG. Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer 2000;28:173-85. [Crossref] [PubMed]
- Strauss GM, Kwiatkowski DJ, Harpole DH, et al. Molecular and pathologic markers in stage I non-small-cell carcinoma of the lung. J Clin Oncol 1995;13:1265-79. [Crossref] [PubMed]
- Ferrigno D, Buccheri G, Biggi A. Serum tumour markers in lung cancer: history, biology and clinical applications. Eur Respir J 1994;7:186-97. [Crossref] [PubMed]
- Tonnies M, Pfannschmidt J, Bauer TT, et al. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg 2014;98:249-56. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Kwok Y, et al. Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer 2003;42:327-33. [Crossref] [PubMed]
- Louie AV, Rodrigues G, Yaremko B, et al. Management and prognosis in synchronous solitary resected brain metastasis from non-small-cell lung cancer. Clin Lung Cancer 2009;10:174-9. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19-23. [Crossref] [PubMed]
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [Crossref] [PubMed]
- Parikh RB, Cronin AM, Kozono DE, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;89:880-7. [Crossref] [PubMed]
- Mussi A, Pistolesi M, Lucchi M, et al. Resection of single brain metastasis in non-small-cell lung cancer: prognostic factors. J Thorac Cardiovasc Surg 1996;112:146-53. [Crossref] [PubMed]
- Granone P, Margaritora S, D'Andrilli A, et al. Non-small cell lung cancer with single brain metastasis: the role of surgical treatment. Eur J Cardiothorac Surg 2001;20:361-6. [Crossref] [PubMed]
- Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg 2012;41:617-22. [Crossref] [PubMed]
- Demange L, Tack L, Morel M, et al. Single brain metastasis of non-small cell lung carcinoma. Study of survival among 54 patients. Br J Neurosurg 1989;3:81-7. [Crossref] [PubMed]
- Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75. [Crossref] [PubMed]
- Yuksel C, Bozkurt M, Yenigun BM, et al. The outcome of bifocal surgical resection in non-small cell lung cancer with synchronous brain metastases: results of a single center retrospective study. Thorac Cardiovasc Surg 2014;62:605-11. [PubMed]
- Melloni G, Bandiera A, Gregorc V, et al. Combined treatment of non-small cell lung cancer with synchronous brain metastases: a single center experience. J Cardiovasc Surg (Torino) 2011;52:613-9. [PubMed]
- Plones T, Osei-Agyemang T, Krohn A, et al. Surgical Treatment of Extrapulmonary Oligometastatic Non-small Cell Lung Cancer. Indian J Surg 2015;77:216-20. [Crossref] [PubMed]
- Kanou T, Okami J, Tokunaga T, et al. Prognosis associated with surgery for non-small cell lung cancer and synchronous brain metastasis. Surg Today 2014;44:1321-7. [Crossref] [PubMed]
- Xu Q, Wang Y, Liu H, et al. Treatment outcome for patients with primary NSCLC and synchronous solitary metastasis. Clin Transl Oncol 2013;15:802-9. [Crossref] [PubMed]
- Kong DS, Lee JI, Nam DH, et al. Prognosis of non-small cell lung cancer with synchronous brain metastases treated with gamma knife radiosurgery. J Korean Med Sci 2006;21:527-32. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Lopez Guerra JL, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys 2012;84:e61-7. [Crossref] [PubMed]
- Chidel MA, Suh JH, Greskovich JF, et al. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Investig 1999;7:313-9. [Crossref] [PubMed]
- Guo G, Lambert P, Ahmed N, et al. Local Treatment Improves Survival in NSCLC Patients With Synchronous Brain Oligometastases. Int J Radiat Oncol Biol Phys 2014;90:S52. [Crossref]
- Girard N, Cottin V, Tronc F, et al. Chemotherapy is the cornerstone of the combined surgical treatment of lung cancer with synchronous brain metastases. Lung Cancer 2006;53:51-8. [Crossref] [PubMed]
- Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2015;91:710-7. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Porte H, Siat J, Guibert B, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg 2001;71:981-5. [Crossref] [PubMed]
- Salah S, Tanvetyanon T, Abbasi S. Metastatectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer 2012;75:9-14. [Crossref] [PubMed]
- Milano MT, Katz AW, Zhang H, et al. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012;83:878-86. [Crossref] [PubMed]
- Downey RJ, Ng KK, Kris MG, et al. A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer 2002;38:193-7. [Crossref] [PubMed]
- Howell GM, Carty SE, Armstrong MJ, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol 2013;20:3491-6. [Crossref] [PubMed]
- Salama JK, Chmura SJ, Mehta N, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res 2008;14:5255-9. [Crossref] [PubMed]
- Hasselle MD, Haraf DJ, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol 2012;7:376-81. [Crossref] [PubMed]
- Magilligan DJ Jr, Duvernoy C, Malik G, et al. Surgical approach to lung cancer with solitary cerebral metastasis: twenty-five years' experience. Ann Thorac Surg 1986;42:360-4. [Crossref] [PubMed]
- Cheufou DH, Welter S, Chalvatzoulis E, et al. Surgery of primary lung cancer with oligometastatic m1b synchronous single brain metastasis: analysis of 37 cases. Thorac Cardiovasc Surg 2014;62:612-5. [Crossref] [PubMed]
- Lo SS, Rodrigues GB, Lock M, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer: in regard to Parikh et al. Int J Radiat Oncol Biol Phys 2014;90:716-7. [Crossref] [PubMed]