Pulmonary metastasectomy: a cross sectional survey
Introduction
In the current era of evidence driven surgical practice, pulmonary metastasectomy is supported only by non-randomised, retrospective case series and metastasectomy registries. Despite its widespread conduct, there are no published clinical guidelines for pulmonary metastasectomy. The goals of this research were to identify important and controversial topics in contemporary pulmonary metastasectomy and conduct an international survey to generate general guidelines for the practice of pulmonary metastasectomy.
The first published case-report of pulmonary metastasectomy was performed in 1939 (1). Since that time, the understanding of cancer biology including patterns of cancer spread, imaging, surgical techniques and adjunctive therapies has broadened immensely. The original resection criteria put forward by Thomford in 1965, emphasised the role of pulmonary metastasectomy to primarily improve survival, whilst also being used palliatively for patients with locally complicated disease (2). Rusch in 1995 described her own set of criteria for pulmonary metastasectomy. These included control of the primary tumour, patient anatomy, physiology and pulmonary reserve sufficient to withstand planned resection, complete metastatic resection, absence of extra-thoracic disease, and surgical therapy when no less morbid, equally effective alternative systemic therapy is available. Rusch’s principles have generally been accepted by many thoracic surgeons internationally over the last two decades (3).
Following on from Thomford and Rusch, and the multitude of retrospective studies that have been published on pulmonary metastasectomy, in 2005 the European Society of Thoracic Surgeons (ESTS) formed a working group and distributed a questionnaire in 2006, surveying surgeons with regards to their current surgical approaches to pulmonary metastasectomy (4). It provided an insight into the patterns of pulmonary metastasectomy across a wide range of clinical practices.
Methods
Questionnaire development
An online literature search was used to identify important and controversial topics in contemporary pulmonary metastasectomy. These topics included the role of surgery, indications and contra-indications to resection, pre-operative patient evaluation, operative strategies, follow up and alternative treatment. The questions were designed in such a way as to lead respondents to give general recommendations.
Invitation to participate
The international experts approached to participate in this study were a pre-formed group of thoracic surgeons who previously contributed to a consensus statement on video-assisted thoracoscopic surgery (VATS) lobectomy (5). They were identified originally in 2012 as representatives of internationals thoracic units who had completed more than 100 VATS lobectomy procedures. No incentives or disincentives were suggested as part of their participation or non-participation.
Voting process
An individualised invitation was emailed to each of experts with a link to a secure website that presented 18 questions. Two rounds of voting were used to strengthen validity. Invitation to the first round of voting was distributed in September 2017. Two reminder e-mails were sent to experts before the first round of voting was closed. An e-mail invitation to view results of the first round of voting and concurrently participate in the second round of voting was distributed in November 2017 and two reminder e-mails subsequently sent. The results from the second round of voting formed the basis for practice recommendations.
Ethics
There was no direct patient contact or review of confidential information required for this study. Ethics committee approval was deemed not required.
Results
Demographic data
Twenty-five international experts from 9 countries completed the first round questionnaire. Twenty-two of these 25 completed the second round questionnaire. Of the respondents who completed the second round questionnaire, 73% were from Europe, 23% were from North America and 4% from Australia.
Recommendations
The survey questions and details of their responses is presented in Table 1. A summary of recommendations for each of the 18 consensus questions is presented in Table 2. Clinical practice was deemed ‘recommended’ if 50–74% of the experts reached agreement and ‘highly recommended’ if 75% or more of the experts reached agreement following the second round of voting. Consensus was reached on the following points: (I) the role of pulmonary metastasectomy is to improve local disease control (91%, ‘highly recommended’;) and improve survival (82%, ‘highly recommended’); (II) favourable histological subtypes for pulmonary metastasectomy include colorectal carcinoma (100%, ‘highly recommended’), osteosarcoma (95%, ‘highly recommended’), soft tissue sarcoma (91%, ‘highly recommended’), renal cell carcinoma (86%, ‘highly recommended’), melanoma (68%, ‘recommended’), head and neck carcinoma (68%, ‘recommended’), and gynaecological (cervical/endometrial/ovarian) cancer (55%, ‘recommended’); (III) primary tumour should be controlled prior to metastasectomy (100%, ‘highly recommended); (IV) both positron emission tomography (PET) and computerized tomography (CT) should be used in pre-operative imaging (82%, ‘highly recommended’); (V) patients should be discussed in a multi-disciplinary meeting (100%, ‘highly recommended’); (VI) tissue biopsy is not recommended prior to resection in patients with suspected pulmonary metastases (86%, ‘highly recommended); (VII) incomplete metastasectomy is a contraindication to surgery (91%, ‘highly recommended’); (VIII) enlarged (>10 mm) or glucose avid lymph nodes should be assessed prior to metastasectomy (73%, ‘recommended’); (IX) patients with progressive disease should be observed prior to metastasectomy (95%, ‘highly recommended’); (X) complete resection is the primary treatment goal (82%, ‘highly recommended’); (XI) VATS is the preferred operative approach (82%, ‘highly recommended’); (XII) metastatic lesions should be identified intra-operatively by visualisation and pre-operative imaging correlation (86%, ‘highly recommended’), manual palpation (86%, ‘highly recommended’) and instrument palpation (50%, ‘recommended); (XIII) thoracotomy is preferred for multiple lesions (64%, ‘recommended’) and preservation of lung function (55%, ‘recommended’); (XIV) Acceptable extent of resection is wedge resection (100%, ‘highly recommended’), segmentectomy (95%, ‘highly recommended’), lobectomy (95%, ‘highly recommended’) and bilobectomy (64%, ‘recommended’); (XV) lymph nodes should be assessed intra-operatively (50%, ‘recommended’); (XVI) repeat CT-chest or PET should be performed 6 months following resection (68%, ‘recommended’); (XVII) re-do metastasectomy is indicated in recurrent disease (100%, ‘highly recommended’); (XVIII) Alternative loco-regional treatments include stereotactic radiotherapy (100%, ‘highly recommended’) and radiofrequency ablation (64%, ‘recommended’).
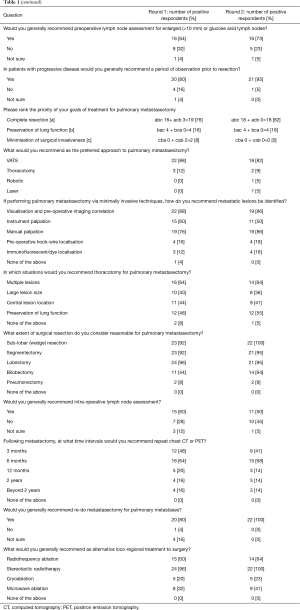
Full table
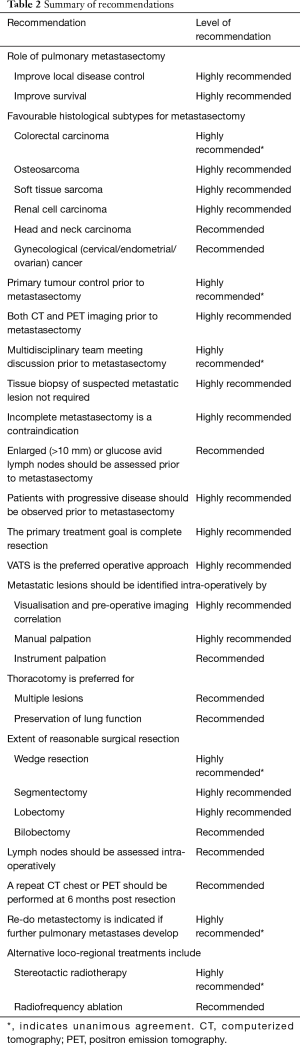
Full table
Discussion
General principles of pulmonary metastasectomy
The role and conduct of pulmonary metastasectomy in contemporary surgical practice remains controversial. Larger clinical series suggest that metastatic tumour resection improves survival (6). In particular, survival benefit is best seen in the context of oligometastatic disease where metastases are completely resected and there is a long disease-free interval from diagnosis of the primary tumour (7). Respondents here agreed that pulmonary metastasectomy is used to improve survival as well as improve local disease control in the context of primary tumour control. Further, complete resection was considered of paramount importance with incomplete resection a contraindication to surgery. Sparing pulmonary parenchyma and limiting surgical invasiveness were considered lesser priorities. It was also agreed that patients with progressive disease be observed prior to metastasectomy, consistent with data suggesting poorer survival in these patients undergoing early resection (8). Tissue biopsy was not generally recommended in the case of suspected metastatic disease.
Histological subtype favourability
Pulmonary metastasectomy represents a potentially curative treatment for patients with metastatic sarcoma, however its role in other histological subtypes is less clear (7,9,10). Interestingly, despite a previous survey of surgeons in Britain and Ireland suggesting equipoise for the resection of colorectal metastases and a randomised control trial currently enrolling patients with colorectal cancer to undergo or not undergo pulmonary metastasectomy, it was unanimously agreed here that colorectal carcinoma is a favourable histological subtype for metastasectomy (11,12). In addition to sarcoma and colorectal carcinoma, renal cell carcinoma, melanoma, gynaecological cancer, and head and neck carcinomas were also considered favourable to resection despite less favourable evidence for the latter (13). Certainly, there is now less favouritism to resect metastatic breast cancer from the lung. This is most likely due to improving systemic therapies effective at prolonging life in the disease (14).
Discussion of patients in a multidisciplinary setting was also unanimously recommended. Multidisciplinary team management has shown to be advantageous in many clinical specialties (15). It affords the opportunity to discuss complimentary and alternate systemic and loco-regional disease control strategies. Targeted, ablative therapies to metastatic pulmonary lesions have increasingly been used instead of surgery and here the preference for stereotactic radiotherapy was again emphasised (16).
Imaging
The quality and application of imaging for metastatic pulmonary disease continues to improve. Computed tomography (CT) of the chest in combination with positron emission tomography (PET) scanning play complimentary roles in identifying the number, location, size and character of pulmonary masses as well as assessment of extra thoracic disease (17,18). The information obtained from these imaging modalities often determines operability and extent of resection. PET has shown to be particularly sensitive in assessment of lesions >10 mm and potentially modifies treatment plans when extra-thoracic disease is discovered (19). The limitations of CT scanning, particularly in missing small malignant nodules has been recognised but likely to be reduced as imaging resolution improves (20). It is perhaps unsurprising that use of both imaging modalities is highly recommended by the expert panel in patients being considered for pulmonary metastasectomy. The role of re-imaging post metastasectomy has not been clearly defined and often longer term follow up is conducted by non-surgical members of the multidisciplinary team (4). Notwithstanding this, following up patients with repeat chest CT at 6 months post resection was recommended by expert consensus of surgeons.
Lymph node assessment
Hilar and mediastinal lymph node involvement is a poor prognostic indicator in pulmonary metastatic disease (21). Despite this, systematic assessment of lymph nodes has historically not been widespread (4) and it is controversial whether patients with positive nodes should be excluded from pulmonary metastasectomy (22). Both preoperative tissue assessment of radiologically suspicious lymph nodes and intra-operative lymph node assessment is ‘recommended’ by the expert panel. Whether nodal positivity would change the management of patients was not determined in the questionnaire.
Operative strategy
Numerous studies have suggested the benefit of less invasive approaches to lung resection in the context of primary lung cancer (23-26). Specifically, VATS has shown to be potentially curative, less painful and expeditious of recovery compared with traditional thoracotomy (27). With advancement of modern thoracoscopic technology and increasingly advanced VATS skills sets, VATS is the favoured approach for pulmonary metastasectomy in this study. The majority support for the approach is contrasted to the 40% support given to VATS as the preferred technique in 2008 (4). Given the panel of experts were original contributors to a consensus statement on VATS lobectomy, it is not surprising that VATS was favoured, although agreement was not unanimous and thoracotomy was considered indicated in the presence of multiple metastatic lesions and for the preservation of lung function.
Lesion localisation in the VATS approach is dependent on a combination of pre-operative imaging and intra-operative palpation. Newer technologies including dye and hook-wire localisation were not recommended in this study. VATS has often been criticised for missing occult metastases and minimising surgical margin compared with thoracotomy (20,28,29). Despite this, numerous authors have shown no difference in operative survival, disease free interval or ipsilateral recurrence between VATS and open approaches (30,31).
The extent of resection, in particular the limit of reasonable resection, has often been discussed. Here the general recommendation to resect lung parenchyma to achieve negative margins up to and including lobectomy was ‘highly recommended’ and up to bilobectomy ‘recommended’. With the increasing sophistication of lung stapler technology, the resection of multiple lesions with minimisation of alveolar air leak has been simplified. The approach to confirmed or suspected bilateral disease was not investigated here.
Re-do pulmonary metastasectomy is sometimes required, particularly for young patients with osteogenic and soft tissue sarcomas where surgery is the preferred disease control strategy (7). With the development of new pulmonary metastases following initial resection, it is ‘highly recommended’ here that patients are considered for further surgery. In the case of ipsilateral chest re-entry, the operative strategy may be different to first time entry. Specifically, minimally invasive approaches may be less appropriate given the presence of pleural adhesions, obscured tissue planes or multiple lesions.
Study limitations
This study has been designed to create a list of general recommendations formulated by an international panel of experts. The expert panel consisting of surgeons previously formed as part of a primary lung cancer VATS lobectomy consensus group. These surgeons are likely to have a more advanced thoracoscopic skill set and the VATS approach to metastasectomy may have been emphasised.
Conclusions
This cross sectional survey establishes general recommendations for the practice of pulmonary metastasectomy. The recommendations are likely to be widely applicable to many clinical circumstances though not intended to replace patient specific cancer care. It is anticipated that it will influence and potentially shape contemporary clinical practice guidelines.
Acknowledgements
I wish to thank the international experts who contributed to the content of this paper. The participants are listed by country and location: Tristan D. Yan (Royal Prince Alfred Hospital, Sydney, Australia); Youri Sokolow (Hospital Erasme, Brussels, Belgium), Georges Decker (University Hospitals Leuven, Leuven, Belgium), Frederic De Ryck (Ghent University Hospital, Ghent, Belgium), Herbert Decaluwe (University Hospitals Leuven, Leuven, Belgium); Nan Wu (Beijing Cancer Hospital and Institute, Beijing, China); Henrik Hansen (Rigshospitalet, Copenhagen, Denmark), Peter Licht (Odense University Hospital, Odense, Denmark), René Horsleben Petersen (Rigshospitalet, Copenhagen, Denmark); Gunda Leschber (ELK Berlin Chest Hospital, Berlin, Germany); Luciano Solaini (S. Maria delle Croci Hospital, Ravenna, Italy); Jan Wolter Oosterhuis (VU Medical Centre, Amsterdam, Netherlands); Sasha Stamenkovic (Freeman Hospital, Newcastle, UK), Tom Routledge (Guys/Thomas’ Hospital, London, UK), Gianluca Casali (University Hospital Bristol NHS Foundation Trust, Bristol, UK), Edwin Woo (Southampton General Hospital, Southampton, UK), Michael Shackcloth (Liverpool Heart and Chest Hospital, Liverpool, UK), Rajesh Shah (Wythenshawe Hospital, Manchester, UK), Joel Dunning (James Cook University Hospital, Middlesbrough, UK); Tommy D’Amico (Duke University Medical Center, Durham, USA), Todd Demmy (Roswell Park Cancer Institute and University at Buffalo, Buffalo, USA), Bernard Park (Hackensack University Medical Center, Hackensack, USA), Scott Swanson (Brigham and Women’s Hospital and the Dana Farber Cancer Institute, Boston, USA), Bryan Meyers (Washington University, St Louis, USA), John Mitchell (University of Colorado School of Medicine, Aurora, USA).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barney JD, Churchill EJ. Adenocarcinoma of the Kidney with Metastasis to the Lung: Cured by Nephrectomy and Lobectomy. J Urol 1939;42:269-76. [Crossref]
- Thomford NR, Woolner LB, Clagett OT. The Surgical Treatment of Metastatic Tumors in the Lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed]
- Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995;107:322S-31S. [Crossref] [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Smith R, Demmy TL. Pulmonary metastasectomy for soft tissue sarcoma. Surg Oncol Clin N Am 2012;21:269-86. [Crossref] [PubMed]
- Krüger M, Schmitto JD, Wiegmann B, et al. Optimal timing of pulmonary metastasectomy--is a delayed operation beneficial or counterproductive? Eur J Surg Oncol 2014;40:1049-55. [Crossref] [PubMed]
- Temple LK, Brennan MF. The role of pulmonary metastasectomy in soft tissue sarcoma. Semin Thorac Cardiovasc Surg 2002;14:35-44. [Crossref] [PubMed]
- Sternberg DI, Sonett JR. Surgical therapy of lung metastases. Semin Oncol 2007;34:186-96. [Crossref] [PubMed]
- Treasure T, Fallowfield L, Lees B. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. J Thorac Oncol 2010;5:S203-6. [Crossref] [PubMed]
- Jegatheeswaran S, Satyadas T, Sheen AJ, et al. Thoracic surgical management of colorectal lung metastases: a questionnaire survey of members of the Society for Cardiothoracic Surgery in Great Britain and Ireland. Ann R Coll Surg Engl 2013;95:140-3. [Crossref] [PubMed]
- Young ER, Diakos E, Khalid-Raja M, et al. Resection of subsequent pulmonary metastases from treated head and neck squamous cell carcinoma: systematic review and meta-analysis. Clin Otolaryngol 2015;40:208-18. [Crossref] [PubMed]
- Welter S, Jacobs J, Krbek T, et al. Pulmonary metastases of breast cancer. When is resection indicated? Eur J Cardiothorac Surg 2008;34:1228-34. [Crossref] [PubMed]
- Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract 2015;11:239-46. [Crossref] [PubMed]
- Siva S, Slotman BJ. Stereotactic Ablative Body Radiotherapy for Lung Metastases: Where is the Evidence and What are We Doing With It? Semin Radiat Oncol 2017;27:229-39. [Crossref] [PubMed]
- Pastorino U, Veronesi G, Landoni C, et al. Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastasis. J Thorac Cardiovasc Surg 2003;126:1906-10. [Crossref] [PubMed]
- Detterbeck FC, Grodzki T, Gleeson F, et al. Imaging requirements in the practice of pulmonary metastasectomy. J Thorac Oncol 2010;5:S134-9. [Crossref] [PubMed]
- Fortes DL, Allen MS, Lowe VJ, et al. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;34:1223-7. [Crossref] [PubMed]
- Cerfolio RJ, McCarty T, Bryant AS. Non-imaged pulmonary nodules discovered during thoracotomy for metastasectomy by lung palpation. Eur J Cardiothorac Surg 2009;35:786-91; discussion 791. [Crossref] [PubMed]
- Ercan S, Nichols FC 3rd, Trastek VF, et al. Prognostic significance of lymph node metastasis found during pulmonary metastasectomy for extrapulmonary carcinoma. Ann Thorac Surg 2004;77:1786-91. [Crossref] [PubMed]
- Menon A, Milton R, Thorpe JA, et al. The value of video-assisted mediastinoscopy in pulmonary metastasectomy. Eur J Cardiothorac Surg 2007;32:351-4. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Sihoe AD. Uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg 2016;5:133-44. [Crossref] [PubMed]
- Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. [Crossref] [PubMed]
- Landreneau RJ, De Giacomo T, Mack MJ, et al. Therapeutic video-assisted thoracoscopic surgical resection of colorectal pulmonary metastases. Eur J Cardiothorac Surg 2000;18:671-6. [Crossref] [PubMed]
- Ellis MC, Hessman CJ, Weerasinghe R, et al. Comparison of pulmonary nodule detection rates between preoperative CT imaging and intraoperative lung palpation. Am J Surg 2011;201:619-22. [Crossref] [PubMed]
- Eckardt J, Licht PB. Thoracoscopic versus open pulmonary metastasectomy: a prospective, sequentially controlled study. Chest 2012;142:1598-602. [Crossref] [PubMed]
- Nakas A, Klimatsidas MN, Entwisle J, et al. Video-assisted versus open pulmonary metastasectomy: the surgeon's finger or the radiologist's eye? Eur J Cardiothorac Surg 2009;36:469-74. [Crossref] [PubMed]
- Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2013;17:720-4. [Crossref] [PubMed]


