Risk factors for noninvasive ventilation failure in patients with post-extubation acute respiratory failure after cardiac surgery
Introduction
Approximately 9–35% of patients develop acute respiratory failure (ARF) after cardiac surgery; the incidence of ARF is higher in patients undergoing complex surgical procedures (1-3). Post-extubation ARF is a serious complication of cardiac surgery and may increase the stay at the intensive care unit (ICU) and in-hospital mortality (4,5).
Studies have reported that the use of noninvasive ventilation (NIV) in patients with ARF may avoid the need for re-intubation after cardiac surgery (2,6). A meta-analysis of trials compared NIV and conventional management after cardiothoracic surgery and showed that the use of NIV improved the patients’ oxygenation and decreased the need for re-intubation. Re-intubation in patients receiving NIV after cardiac surgery was observed in 6–52% of cases (7,8). However, evidence also indicates that the use of NIV in patients with ARF did not decrease the incidence of re-intubation and in-hospital mortality (9,10). Emergent re-intubation after NIV failure may result in significant hemodynamic instability, increasing the risk of nosocomial infections, mortality, and stay at the ICU (11,12).
Numerous studies have suggested that NIV is useful in certain patient populations (5,7,13). Identifying patients with a considerable risk of NIV failure may alert clinicians early that conventional mechanical ventilation might be appropriate. An interval of <24 hours from extubation to NIV has been identified as a risk factor for NIV failure (10). A randomized controlled single-center trial examined the predictors of NIV failure in patients with ARF after extubation and concluded that an Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score >20 was an independent risk factor for noninvasive positive pressure ventilation (14). Further research is required to accurately determine the predictors of NIV failure.
The primary objective of this study was to estimate in-hospital mortality and the length of the stay at the ICU in patients who developed ARF and were initially treated with NIV after cardiac surgery. The secondary objective was to identify the risk factors for NIV failure in patients with post-extubation ARF.
Methods
Study population
We conducted a retrospective observational study at a 29-bed ICU of an academic hospital between March 2013 and April 2014. Data were collected from patients who underwent cardiac surgery and developed ARF after extubation. Patients receiving NIV only during procedural sedation and those receiving NIV to prevent ARF were excluded. This study was approved by the Committee on Ethics of Biomedicine Research, Second Military Medical University, Shanghai (No. SMMUEC2018-012).
ARF was diagnosed when a patient satisfied one or both the following criteria: (I) arterial partial pressure of carbon dioxide (PaCO2) ≥50 mmHg or arterial partial pressure of oxygen (PaO2) <80 mmHg with a fraction of inspired oxygen (FiO2) >40%; (II) evidence of moderate to severe respiratory distress, tachypnea (respiratory rate >30 breaths/min, use of accessory muscles for respiration).
Data collection
The following data were collected for each patient: age, sex, weight, height, body mass index (BMI), overweight (BMI ≥25 kg/m2), ventricular ejection fraction (EF), duration of NIV, associated diagnoses, and data for calculating the EuroSCORE (European System for Cardiac Operative Risk Evaluation). The duration of NIV was defined as the time from the initiation of NIV to either re-intubation or successful transition to breathing through a nasal cannula. The following data were collected before commencing NIV: heart rate, respiratory rate, vasoactive-inotropic score {dosages of dopamine + dobutamine (µg/kg/min) + [dosages of epinephrine + norepinephrine + isoproterenol (µg/kg/min)] ×100 + dosages of milrinone (µg/kg/min) ×15} (15), time elapsed since extubation (in hours), days of intubation before extubation, dysfunction involving two or more organ systems, pneumonia or sepsis (culture-proven or suspected), arterial pH, FiO2, PaCO2, PaO2, and white blood cell count.
Extubation criteria
Tracheal extubation was performed according to a standardized protocol for ventilator weaning used in our ICU. Patients were extubated when they met the following criteria: awake, cooperative, with no evidence of neurological deficits; stable hemodynamic status with no requirement for high-dose vasoactive-inotropic support; urine output >0.5 mL/kg/h; no severe arrhythmia; PaO2 >80 mmHg with FiO2 <40% and positive end-expiratory pressure ≤5 cmH2O; normal PaCO2 after a trial of pressure support of ≤10 cmH2O for at least 30 minutes.
NIV technique
Two methods of NIV were used in this study: continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BIPAP). The decision of whether to use CPAP or BIPAP was made by the ICU attending physician. Three types of ventilators were used in this study: EVITA 4 (Drägerwerk, Lübeck, Germany), Elisee 350 (ResMed, New Zealand, Australia), and Vela (Bird, Caspian, USA). The PSV mode was used for NIV treatment. The NIV was administered by a dual-limb Y ventilator circuit, consisting of an inspiratory limb, a patient connector, and an expiratory limb. A nasogastric tube was inserted in all patients before the initiation of NIV to prevent gastric distension and emesis.
Re-intubation criteria
The decision to re-intubate was made by the ICU attending physician and the team caring for the patient. Possible reasons for re-intubation included cardiorespiratory arrest, hemodynamic decompensation, inability to clear the airway or oral secretions, NIV intolerance (due to difficulty tolerating the facial mask or lack of cooperation), worsening hypoxemia (decrease of ≥20% from baseline), hypercarbia (increase of ≥20% from baseline), clinical signs of respiratory fatigue and severe respiratory distress despite maximum NIV support, and a Glasgow Coma Scale <8 or inability to maintain adequate airway patency due to neurologic impairment.
End points and definitions
The primary outcome variables were the need for endotracheal intubation and NIV-related in-hospital mortality.
The causes of ARF were classified as follows: acute cardiogenic edema (cardiac index <1.8 L/min/m2, pulmonary capillary wedge pressure >18 mmHg and high-dosage catecholamines, intra-aortic balloon pump, postoperative myocardial infarction, or severe arrhythmia); pneumonia (positive microbiology culture and appropriate imaging and clinical features, including a temperature of >38.5 or <35 °C and white blood cell count >11×109 or <4×109 cell/L); post-cardiopulmonary bypass (CPB) lung impairment (according to the acute lung injury criteria without cardiogenic compromise and negative “pneumonia” criteria) (16); lobar atelectasis; reactive airway disease; neuromuscular disease; hypercapnic pump failure; and other conditions (including pneumothorax and shock). Three types of NIV failure were identified according to the time of failure: immediate failure (within 1 hour), early failure (1–48 hours), and late failure (after 48 hours) (17). The reasons for NIV failure included: (I) the inability to correct the gas exchange; (II) a weak cough reflex and/or excessive secretions; (III) hemodynamic instability; (IV) intolerance and psychomotor agitation; (V) hypercapnic encephalopathy and coma; (VI) surgical re-exploration; and (VII) refractory hypoxemia due to infection.
Statistical analysis
Continuous variables are presented as means ± standard deviations or medians and interquartile ranges. Comparisons of continuous variables between the two groups were performed with the Student’s t-test for variables with a normal distribution. The Wilcoxon rank sum test was used for variables with a non-normal distribution. Categorical variables were analyzed using the chi-squared test, and the results are presented as proportions with 95% confidence intervals (CI).
The cutoff value for continuous variables were obtained using receiver operating characteristic (ROC) curves. Binary univariate and multivariate logistic regression analyses were performed to identify the risk factors for NIV failure. Significant variables associated with NIV failure in the univariate analysis were included in the multivariate analysis. The multivariate regression analysis used a forward stepwise (conditional) procedure to determine the independent significant prognostic factors.
All P values were two-sided. Statistical significance was defined as P<0.05. All statistical analyses were performed with the SAS software (version 9.3, SAS Institute, Inc., Cary, NC, USA).
Results
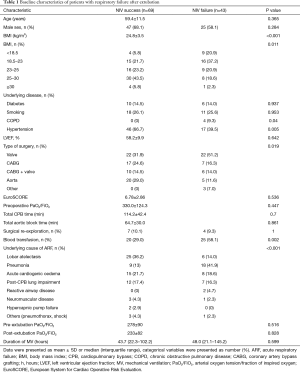
A total of 1,713 adult patients were enrolled in this study. ARF developed after initial extubation in 126 patients; of these, 14 did not undergo NIV because they required re-intubation because of their clinical condition. Finally, 112 patients were treated with NIV for ARF after initial extubation (Figure 1). Of these patients, 43 were in the NIV failure and 69 in the NIV success group. Table 1 presents the characteristics and medical history of the NIV success and failure groups. No differences in the EuroSCORE and preoperative PaO2/FiO2 were seen between the two groups. In the NIV success group, the BMI was higher; fewer patients had chronic obstructive pulmonary disease and more had hypertension.
Full table
The median interval between NIV treatment and re-intubation was 19 hours 45 minutes (interquartile range, 4 hours 15 minutes to 70 hours). Most cases of NIV failure occurred in the early period (1–48 hours after NIV treatment; Figure 2). The causes of early failure included the inability to correct the gas exchange (3 patients), hemodynamic instability (12 patients), and a weak cough reflex and/or excessive secretion (9 patients). The reasons for late failure (after 48 hours) were refractory hypoxemia because of infection (5 patients) and a weak cough reflex and/or excessive secretion (3 patients).
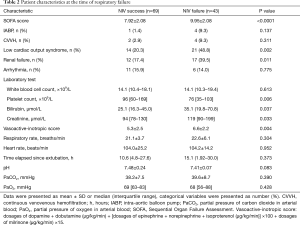
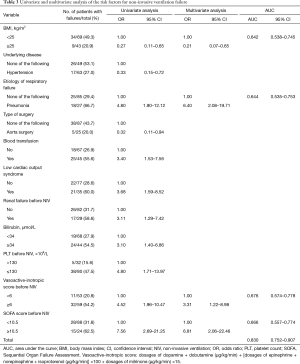
The etiology of ARF in patients who underwent NIV treatment is shown in Table 1. Endotracheal intubation was required in 10/27 patients with pneumonia, 37% of the 19 patients with post-CPB lung impairment, and 35% of the 23 patients with acute cardiogenic edema. The lowest intubation rate (19%) was observed among patients with lobar atelectasis. Table 2 compares the characteristics between the NIV success and failure groups at the time of respiratory failure. NIV failure was associated with a higher Sequential Organ Failure Assessment (SOFA) score (Figure 3) and vasoactive-inotropic score, higher creatinine levels, and lower platelet counts; it occurred more frequently in patients with low cardiac output syndrome and renal failure.
Full table
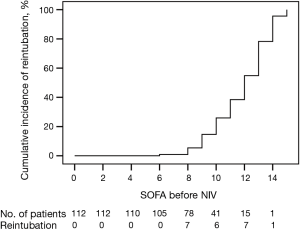
To predict the risk factors of NIV failure, the eleven variables that showed significant differences in the univariate analysis (pneumonia, SOFA score ≥10.5, low cardiac output syndrome, renal failure, transfusion of blood products >30 units, vasoactive-inotropic score ≥6, platelet count <130×109/L, bilirubin ≥34 µmol/L, BMI ≥25 kg/m2, hypertension, and aortic surgery) were analyzed in the binary logistic multivariate regression analysis. A SOFA score ≥10.5 (OR: 6.8, 95% CI: 2.06–22.46, P=0.002), vasoactive-inotropic score ≥6 (OR: 3.31, 95% CI: 1.22–8.98; P=0.019), and pneumonia (OR: 6.40, 95% CI: 2.08–19.71; P=0.001) were independent predictive factors for NIV failure, whereas a BMI ≥25 kg/m2 was an independent predictive factor for NIV success (OR: 0.21, 95% CI: 0.07–0.65; P=0.007) (Table 3).
Full table
A prognostic model based on the independent prognostic factors in the multivariate analysis was developed and calculated as follows: −1.586−1.561× (BMI ≥25) +1.918× (SOFA score ≥10.5) +1.857× pneumonia +1.198× (vasoactive-inotropic score ≥6). The overall model performance was satisfactory, with an AUROC of 0.830 (95% CI: 0.752–0.907) (Table 3).
The in-hospital mortality rate was significantly higher in the NIV failure than in the NIV success group (44.2% vs. 1.4%; P<0.0001; Table 4). The length of stay at the ICU in patients with NIV failure was significantly longer than in those with NIV success [17.23 (10.07–31.83) vs. 6.68 (4.72–10.91) days, respectively; P<0.0001].
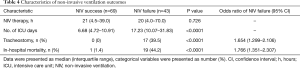
Full table
Discussion
The main findings of this study were that approximately one-third of patients (38.4%) treated with NIV for moderate or severe ARF after extubation failed NIV, ultimately requiring invasive ventilation with an associated mortality of 44%, compared to 1% for NIV success and a length of stay at the ICU of 17 days, compared to 7 days for NIV success. A SOFA score ≥10.5, vasoactive-inotropic score ≥6, and pneumonia before NIV were predictive factors of NIV failure in patients with post-extubation ARF, while a BMI ≥25 kg/m2 was a predictor of NIV success.
A SOFA score ≥10.5 was a strong predictor of NIV failure in patients with post-extubation ARF after cardiac surgery in the current study. The SOFA score is used to diagnose multiple organ failure and predict fatal outcomes in critically ill patients (18,19). It can also be used to predict mortality and morbidity after cardiac surgery (20,21). A previous study found that the SOFA score was an independent risk factor for mortality of patients who underwent isolated coronary artery bypass grafting (CABG) (OR, 2.036; 95% CI: 1.685–2.460, P<0.001) (22). However, to the best of our knowledge, no study has examined the association between SOFA scores and NIV use in patients with ARF after cardiac surgery. In our study, the pre-NIV SOFA score was associated with the re-intubation rate. The reason for this higher reintubation rate may be that patients in the NIV failure group had higher serum bilirubin levels; moreover, a higher proportion of patients in the NIV failure group developed renal failure or cardiac output syndrome, implying that more patients in the NIV failure group had multiple organ failure. Furthermore, the higher SOFA score, the more fragile patients are (18). In our study, the SOFA score predicted the NIV outcome with high sensitivity and accuracy; therefore, NIV should not be used in patients with a SOFA score ≥10.5.
In our study, we also found that pneumonia was an independent predictive factor for NIV failure. In an observational prospective study, the presence of pneumonia was a predictor of NIV failure in patients with chronic obstructive pulmonary disease (23). Our study found that once pneumonia occurred before NIV, re-intubation was inevitable in 66.7% of the patients. This is consistent with previous reports showing that pneumonia reduced the efficiency of the gas exchange, causing hemodynamic instability (24). Pneumonia was associated with a significantly increase in mortality (HR, 8.89; 95% CI: 5.02–15.75) and length of hospital stay (13.6±2.0 days) in a prospective multicenter study of 5,158 adult patients undergoing cardiac surgery (25). Patients with pneumonia after cardiac surgery may have excessive secretions and may not respond well to NIV because of fever and fragility. Therefore, in patients with post-extubation ARF after cardiac surgery, pneumonia might be a contradiction for NIV.
BMI also affected the NIV treatment outcome in this report. Appling NIV immediately after extubation in obese patients (defined as a BMI ≥35 kg/m2) could be effective for predicting ARF (26). In patients having undergone cardiac surgery, obesity (defined as a BMI >30 kg/m2) was a predictive factor for NIV success (10). Our study revealed that patients with a BMI ≥25 kg/m2 had a higher rate of NIV success than those with a BMI <25 kg/m2 (49% vs. 21%, respectively, P=0.003). We assume that the reason for the higher success rate is that more patients with overweight (BMI ≥25 kg/m2) showed hypercapnia (PaCO2 >50 mmHg) than those with normal weight (11.6% vs. 0%, P=0.023) and had lower vasoactive-inotropic scores [5.6 (4.2–6.7) vs. 6.3 (5.1–7.6); P=0.010] pre-NIV. Overweight patients may be at increased risk of alveolar collapse and thus, NIV is very useful in these patients.
The vasoactive-inotropic score is a predictor of morbidity and mortality in infants and adults after cardiac surgery (27-29). After adjusting for the EuroSCORE, preoperative EF, and bypass time, a high vasoactive-inotropic score after surgery was associated with a poor outcome, longer stay at the ICU, and longer ventilation support in adult cardiac surgery patients (30). Our study found that a vasoactive-inotropic score ≥6 was a risk factor of NIV failure in patients with post-extubation ARF after cardiac surgery. A higher vasoactive-inotropic score might mean low cardiac output syndrome or sepsis, and a persistent requirement of vasoactive agents may be a marker of disease severity (31). The vasoactive-inotropic score could be useful in identifying the marginal circulation stage before low cardiac output syndrome or sepsis are diagnosed. Thus, vasoactive-inotropic scores should be carefully evaluated before NIV use.
The main limitation of our study was its retrospective study and that it was conducted at a single ICU, causing inevitable selection bias. A prospective randomized controlled trial may provide more reliable results. In this study we mainly focused on the parameter before NIV use. Moreover, other variables known to be useful to assess the risk of NIV failure, such as B-type natriuretic peptide levels and fluid balance, were not analyzed. High flow nasal cannula oxygen (HFNC) has been proven useful in the treatment of ARF after cardiac surgery. At our ICU, HFNC was implemented in 2014. Future studies should compare the effects of NIV and HFNC in patients with post-extubation ARF after cardiac surgery.
In conclusion, our study found that NIV was successful in approximately two-thirds of patients after cardiac surgery with moderate or severe ARF after extubation. NIV failure was associated with increased mortality and a longer stay at the ICU. Multiple organ dysfunction, a high vasoactive-inotropic score, and pneumonia before NIV were independent risk factors of NIV failure, whereas overweight was associated with NIV success.
Acknowledgements
Funding: This study was supported by the Wu Jieping Medical Foundation (reference number 320.6750.16093) and Natural Science Foundation of Shanghai (reference number 16ZR1400900).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Committee on Ethics of Biomedicine Research, Second Military Medical University, Shanghai (No. SMMUEC2018-012).
References
- Filsoufi F, Rahmanian PB, Castillo JG, et al. Predictors and early and late outcomes of respiratory failure in contemporary cardiac surgery. Chest 2008;133:713-21. [Crossref] [PubMed]
- Kilger E, Mohnle P, Nassau K, et al. Noninvasive mechanical ventilation in patients with acute respiratory failure after cardiac surgery. Heart Surg Forum 2010;13:E91-5. [Crossref] [PubMed]
- Luo Z, Han F, Li Y, et al. Risk factors for noninvasive ventilation failure in patients with acute cardiogenic pulmonary edema: A prospective, observational cohort study. J Crit Care 2017;39:238-47. [Crossref] [PubMed]
- Canver CC, Chanda J. Intraoperative and postoperative risk factors for respiratory failure after coronary bypass. Ann Thorac Surg 2003;75:853-7; discussion 857-8. [Crossref] [PubMed]
- Zhu G, Huang Y, Wei D, et al. Efficacy and safety of noninvasive ventilation in patients after cardiothoracic surgery: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Nakazato K, Takeda S, Tanaka K, et al. Aggressive treatment with noninvasive ventilation for mild acute hypoxemic respiratory failure after cardiovascular surgery: retrospective observational study. J Cardiothorac Surg 2012;7:41. [Crossref] [PubMed]
- Pieczkoski SM, Margarites AG, Sbruzzi G. Noninvasive Ventilation During Immediate Postoperative Period in Cardiac Surgery Patients: Systematic Review and Meta-Analysis. Braz J Cardiovasc Surg 2017;32:301-11. [PubMed]
- Cabrini L, Zangrillo A, Landoni G. Preventive and therapeutic noninvasive ventilation in cardiovascular surgery. Curr Opin Anaesthesiol 2015;28:67-72. [Crossref] [PubMed]
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004;350:2452-60. [Crossref] [PubMed]
- Garcia-Delgado M, Navarrete I, Garcia-Palma MJ, et al. Postoperative respiratory failure after cardiac surgery: use of noninvasive ventilation. J Cardiothorac Vasc Anesth 2012;26:443-7. [Crossref] [PubMed]
- Boeken U, Schurr P, Kurt M, et al. Early reintubation after cardiac operations: impact of nasal continuous positive airway pressure (nCPAP) and noninvasive positive pressure ventilation (NPPV). Thorac Cardiovasc Surg 2010;58:398-402. [Crossref] [PubMed]
- Beverly A, Brovman EY, Malapero RJ, et al. Unplanned Reintubation Following Cardiac Surgery: Incidence, Timing, Risk Factors, and Outcomes. J Cardiothorac Vasc Anesth 2016;30:1523-9. [Crossref] [PubMed]
- Yang Y, Liu N, Sun L, et al. Noninvasive Positive-Pressure Ventilation in Treatment of Hypoxemia After Extubation Following Type-A Aortic Dissection. J Cardiothorac Vasc Anesth 2016;30:1539-44. [Crossref] [PubMed]
- Zhu GF, Wang DJ, Liu S, et al. Efficacy and safety of noninvasive positive pressure ventilation in the treatment of acute respiratory failure after cardiac surgery. Chin Med J (Engl) 2013;126:4463-9. [PubMed]
- Ko WJ, Lin CY, Chen RJ, et al. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg 2002;73:538-45. [Crossref] [PubMed]
- De Santo LS, Bancone C, Santarpino G, et al. Noninvasive positive-pressure ventilation for extubation failure after cardiac surgery: Pilot safety evaluation. J Thorac Cardiovasc Surg 2009;137:342-6. [Crossref] [PubMed]
- Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med 2014;14:19. [Crossref] [PubMed]
- Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754-8. [Crossref] [PubMed]
- Forstner C, Lassnigg A, Tobudic S, et al. A prospective analysis of invasive candidiasis following cardiac surgery: severity markers are predictive. J Infect 2013;66:528-35. [Crossref] [PubMed]
- Badreldin AM, Doerr F, Ismail MM, et al. Comparison between Sequential Organ Failure Assessment score (SOFA) and Cardiac Surgery Score (CASUS) for mortality prediction after cardiac surgery. Thorac Cardiovasc Surg 2012;60:35-42. [Crossref] [PubMed]
- Badreldin A, Elsobky S, Lehmann T, et al. Daily-Mean-SOFA, a new derivative to increase accuracy of mortality prediction in cardiac surgical intensive care units. Thorac Cardiovasc Surg 2012;60:43-50. [Crossref] [PubMed]
- Chang CH, Chen SW, Fan PC, et al. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg 2017;17:22. [Crossref] [PubMed]
- Pacilli AM, Valentini I, Carbonara P, et al. Determinants of noninvasive ventilation outcomes during an episode of acute hypercapnic respiratory failure in chronic obstructive pulmonary disease: the effects of comorbidities and causes of respiratory failure. Biomed Res Int 2014;2014. [Crossref] [PubMed]
- Ferrer M, Torres A. Noninvasive ventilation for acute respiratory failure. Curr Opin Crit Care 2015;21:1-6. [Crossref] [PubMed]
- Ailawadi G, Chang HL, O'Gara PT, et al. Pneumonia after cardiac surgery: Experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg 2017;153:1384-91.e3. [Crossref] [PubMed]
- El-Solh AA, Aquilina A, Pineda L, et al. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006;28:588-95. [Crossref] [PubMed]
- Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234-8. [Crossref] [PubMed]
- Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014;15:529-37. [Crossref] [PubMed]
- Barge-Caballero E, Segovia-Cubero J, Gonzalez-Vilchez F, et al. Evaluation of the preoperative vasoactive-inotropic score as a predictor of postoperative outcomes in patients undergoing heart transplantation. Int J Cardiol 2015;185:192-4. [Crossref] [PubMed]
- Yamazaki Y, Oba K, Matsui Y, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018;32:167-73. [Crossref] [PubMed]
- Sanil Y, Aggarwal S. Vasoactive-inotropic score after pediatric heart transplant: a marker of adverse outcome. Pediatr Transplant 2013;17:567-72. [Crossref] [PubMed]




