Tubeless single-port thoracoscopic sublobar resection: indication and safety
Introduction
The development of video-assisted thoracoscopic surgery (VATS) in the past two decades has been revolutionary. The concept of minimally invasive procedures has persistently led the thoracic surgeon to a goal of less trauma, less pain, and faster recovery. Several modifications have been applied to VATS to achieve this goal. Early removal of chest tube, or even avoiding chest tube insertion after thoracic surgery, are the earliest modifications to decrease postoperative pain and expedite postoperative recovery (1,2). Single-port VATS and VATS without endotracheal intubation (non-intubated VATS) are two additional modifications that have emerged as representative of clinical innovations of VATS in the past decade (3-5).
Today, single-port VATS for major pulmonary resection has been proven to be comparable with conventional VATS in terms of feasibility and safety (6,7); non-intubated VATS, to minimize the side-effects of intubation-related complications, has also been proven feasible for various VATS procedures (8,9). Thoracic surgery without chest drainage in selected patients has been adopted for more than one decade (2,10-14). However, few studies have combined the above-mentioned three modifications in the tubeless single-port VATS technique for the management of thoracic diseases (15-17).
One of the concerns about omitting chest drainage after VATS is residual pneumothorax, which may require re-insertion of the chest drain if clinically significant (10,12,13). Although the incidence of postoperative pneumothorax has been reported as ranging from 7.6% to approximately 20% in the literature, a relatively higher percentage of postoperative pneumothorax (59%) has been seen in a previous study (11-14). In the non-intubated setting, pulmonary recruitment with mask-ventilation and the water-seal air leak test at the end of surgery is relatively operator-dependent. The reported postoperative residual pneumothorax can be as high as 40% (17). Therefore, the safety of the tubeless VATS technique remains to be challenged. In the present study, we set up a protocol to select patients for tubeless single-port VATS with monitoring of a digital drainage system (DDS) (Thopaz, Medela Healthcare, Switzerland). We present our initial results of this protocol-selected cohort for tubeless single-port technique for pulmonary resection.
Methods
Patients and study design
This was a retrospective study reviewing a prospectively collected database of patients who had undergone single-port VATS at the Taipei Veterans General Hospital (VGH). The study was approved by the institutional review board of Taipei VGH (approval number: 2017-01-016AC).
We began practice of single-port VATS for major and minor pulmonary resections in March 2013, and started non-intubated VATS in November 2016. In order to further minimize postoperative pain and expedite recovery, we designed a protocol to select patients for whom intercostal drainage could be omitted after single-port non-intubated VATS (tubeless VATS). We intentionally included patients with small and peripheral pulmonary lesions without preoperative pathologic diagnosis for sublobar resection, which included a majority of wedge resections. VATS was for diagnostic or curative intent. The selection criteria for the tubeless VATS protocol were: peripheral pulmonary lesion ≤2 cm, no obstructive ventilatory defect with forced expiratory volume in one second (FEV1) ≥1.5 L, and no intrapleural adhesion observed under thoracoscopic vision. Patients who had pleural diseases such as mesothelioma, malignant or infectious pleural effusion, or secondary or primary pneumothorax were excluded. Patients who have a pathologically proven diagnosis of malignancy requiring lobectomy, sublobar resection of ≥2 anatomic segments, ≥3 separated wedge resections, or mediastinal lymph node dissections of ≥3 N2 stations were also excluded (Figure 1).
Non-intubated anesthesia techniques
Patients were pre-medicated with intravenous midazolam (2 mg) and alfentanil (400 mcg). A thoracic epidural catheter was placed at the T7-8 level and tested for efficacy. An intra-epidural single-bolus injection of 0.375% bupivacaine 10 mL and 50 mcg fentanyl was given. After placing each patient in the decubitus position, propofol was given by a target-controlled infusion pump (Agilia, SB Medica SRL, Italy). A bispectral index sensor (Aspect Medical System, Norwood, MA) was used to monitor the level of consciousness. During the procedure, patients breathed spontaneously with a high-flow nasal cannula (Thrive, Fisher & Paykel Healthcare, Auckland, New Zealand).
Single-port techniques
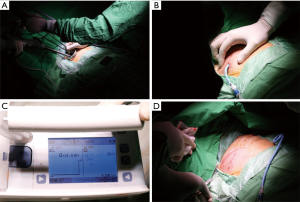
A single 3-cm incision was made in the fifth intercostal space along the anterior axillary line, and a wound retractor (LapShield, Lagis, Taiwan) was used without rib spreading. Lung collapse was obtained by iatrogenic pneumothorax and gentle compression with endoscopic sponges while the patient remained spontaneously breathing. All procedures were performed using thoracoscopic assistance, in which a 5- or 10-mm 30-degree thoracoscopic video camera, grasping instruments, and mechanical staplers were simultaneously fitted into the single incision (Figure 2A). At the end of the procedure, intercostal nerve blocks were performed under thoracoscopic guidance.
Selected patients with chest drainage omitted per the protocol
Among those who met the tubeless protocol criteria, a single 16-Fr pigtail catheter was placed into the pleural cavity and connected to a DDS in which pressure was maintained at −15 cmH2O. At the end of surgery, before the wound was closed it was covered with wet gauze and the lung was re-expanded using negative intrapleural pressure provided by the DDS, and the re-expansion process was fully examined under thoracoscopic vision. The single incision was then closed continuously (Figure 2B). If the air flow reached zero before completion of wound closure, the pigtail catheter was removed immediately (Figure 2C,D); otherwise, the pigtail catheter was kept in place for postoperative drainage purposes. Because this is a per protocol analysis, we included all the patients who met the criteria of “tubeless protocol” for analysis, irrespective of status that the pigtail catheter was kept or removed at the end of surgery.
Postoperative monitoring and medications
Chest roentgenograms were obtained at postoperative 1 hour and postoperative day 1 to ensure lung expansion. Most of our patients were discharged home on postoperative day 2. After being discharged home, medications for pain were not routinely prescribed but were based on each patient’s need.
Results
From March 2013 to September 2017, 316 consecutive patients who underwent single-port VATS pulmonary resections in our institute were reviewed. We started our single-port VATS technique in Mar 2013. Since we adopted a non-intubated general anesthesia setting for VATS in November 2016, we combined the two techniques (non-intubated single-port VATS) in 50 consecutive patients who underwent pulmonary resections. For those who underwent non-intubated single-port VATS and met the criteria for the tubeless protocol, we selected 36 patients in whom we attempted to avoid chest drainage after surgery. Among these 36 patients, the air flow detected by DDS could not reach zero in five patients, who were considered to have a minor air leak or residual air space due to inadequate pulmonary expansion. Therefore, a chest drainage tube was kept for postoperative drainage. Omitting chest drainage after surgery was successfully done in the remaining 31 patients (Figure 1).
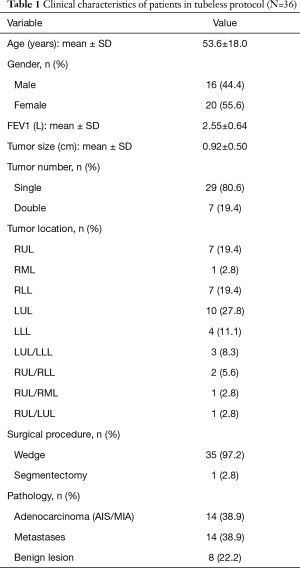
Because our study is a per-protocol analysis, we included 36 patients for whom omitting chest drain was attempted. Among the 36 protocol cases, 8 patients had pulmonary tumors that were benign (22.2%), 14 had adenocarcinoma (38.9%), and 14 had metastatic carcinoma (38.9%); all were managed with sublobar resections with a majority of wedge resections (including 35 wedge resections and 1 right superior segmentectomy). The average tumor size was 0.92±0.50 cm, with 7 patients having two synchronous pulmonary tumors resected over different ipsilateral (6 patients) or bilateral (1 patient) lobes (Table 1).
Full table
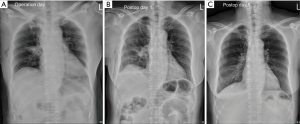
For the 36 patients eligible for the tubeless technique, the postoperative hospital stay was 2.5±0.8 days. Interestingly, the chest roentgenograms taken on the operation day (postoperative 1 hour) showed no pneumothorax (Figure 3A), while 7 (19.4%) patients were noted to have minor pneumothorax on the chest roentgenograms taken on postoperative day 1 (Figure 3B). However, none received additional intervention and all had no residual pneumothorax or pleural effusion at the first outpatient visit (Figure 3C). Remarkably, only 3 (8.3%) required oral analgesics after being discharged (Table 2).
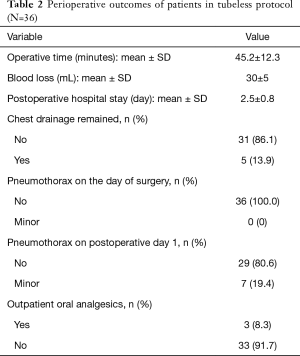
Full table
For the 5 patients who required intercostal drainage because DDS failed to reach 0 mL/min, the average length of time needed for postoperative chest drainage was 2.6 days (Table 3). No pneumothorax or residual pleural effusion was seen in later chest roentgenograms on the operation day or postoperative day 1 among these patients. The average postoperative hospital stay was 3.6 days, and they were all discharged uneventfully.

Full table
Discussion
VATS is undoubtedly the most revolutionary change in thoracic surgery of this generation. However, conventional VATS, with creation of three or four ports under general anesthesia with double-lumen endotracheal intubation and postoperative intercostal drainage for days, has been modified in several ways in the past decade leading to the current “Modified VATS”. The modified VATS include features such as a single-port technique, or general anesthesia without endotracheal intubation (non-intubated VATS), or omitting intercostal drainage (Figure 4). The modifications being made for conventional VATS further minimize postoperative pain, lower the complication rate, and expedite recovery. In the literature, combinations of the single-port technique and non-intubated general anesthesia have been reported (18,19). Omitting chest tubes after conventional VATS in selected patients has also been proven feasible in previous studies (10-14). However, the tubeless single-port VATS (Figure 4), with the combination of the single-port technique, non-intubated general anesthesia, and omitting the chest tube after surgery, has emerged as a more attractive procedure, and has been attempted in some institutions with preliminary results (16,17).
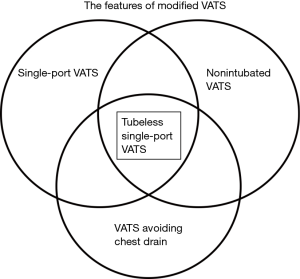
The rationale for proposing the tubeless VATS is to further minimize the trauma associated with tube interventions, to optimize the patient’s experience, and to enhance postoperative recovery (16). However, critics may still be concerned about the complications, especially postoperative air leaks that may cause clinically significant pneumothorax requiring intervention. To minimize the risk of clinically significant pneumothorax, we discuss three specific aspects: selection, prevention, and detection.
First is to select the appropriate candidates for tubeless single-port VATS. The criteria for tubeless single-port VATS in the reported literature are: (I) body mass index <25 kg/m2; (II) solitary pulmonary nodules that can be treated with sublobar resection or peripherally located tumors smaller than 2 cm; (III) an absence of obstructive ventilatory defects; (IV) an absence of severe pleural adhesions; (V) no unfavorable airway features; and (VI) wedge resection of less than 12 cm in the longest dimension of the resected lung (16,17). Interestingly, wedge resection in certain reports was considered to be a risk factor for postoperative air leak. One study limits the number of wedge resections to ≤2, and Ueda et al. even excluded wedge resections for omitting chest tube after VATS (14,20,21). In our cohort, we chose the patients with peripheral lesions ≤2 cm, with no obstructive ventilatory defect (FEV1 ≥1.5 L), and with no pleural adhesions for sublobar resections. We believe the Endo GIA™ staplers that we use for wedge resection can ensure the integrity of cutting the edge of the lung. We excluded patients with wedge resections in ≥3 separate locations and limited the resection size to <2 anatomic segments as a larger residual pleural space would be expected if a larger portion of lung was resected. However, a study of major pulmonary resection under intubated general anesthesia omitting the chest tube has been reported (21). With more experience, tubeless single-port VATS for major pulmonary resection (lobectomy) in selected patients may be feasible. The criteria are subject to change over time.
Second is prevention of air leak in VATS. Some materials have been reported to minimize the postoperative air leak. To minimize it, bioabsorbable mesh, such as a polyglycolic acid mesh, or application of fibrin glue, have been used (20,22,23). In our study, we included Endo GIA™ with Tri-Staple™ Technology for some of our pulmonary wedge resections, which is known to offer a more secure staple suture formation (24). However, there were still 7 patients who developed pneumothorax on postoperative day 1. We speculated that there must be an additional minor air leak from the staple suture after pulmonary expansion, especially when patients cough. Further enhancement of the staple suture should be considered. The polyglycolic acid sheet was also proven to prevent postoperative leakage after lung resection (25). We believe that proper use of these materials and/or technology can effectively provide better pneumostasis after VATS.
Third is detection of any air leak in surgery. Several methods for detection of air leak have been reported. Traditionally, a sealing test has been done with the lung immersed in warm sterile saline, ventilated, and then observed. Additionally, a suction-induced sealing test has been done by inserting a silicon chest tube into the pleural cavity, temporarily closing the wounds, then placing the chest tube under water and examining it for an air leak while ventilating the patient at 15–20 cmH2O of maximum inspiratory pressure (10,15,16). Some surgeons even connected the chest drain to a vacuum ball in order to select patients for tubeless thoracoscopic procedures (17). However, in a non-intubated anesthesia setting, recruitment maneuvers to re-expand the lung by mask-ventilation are relatively inconsistent and operator-dependent compared with those performed under the setting of general anesthesia with an endotracheal tube in the airway. Furthermore, both under water or vacuum tests are subjective measurements without objective parameters. In our study, our method utilizes a DDS to select patients for tubeless thoracoscopic procedures. Full expansion of the collapsed lung and zero air leakage were ensured at the end of procedure. The advantages include: (I) digitalized evaluation for air leaks and the timing for removal of the catheter; (II) constant and steady negative pleural pressure during lung re-expansion; and (III) controllable inflation and deflation of the lung without the need for recruitment maneuvers by the anesthesiologist (26). We believe that this is a safer and more scientific way to decrease the likelihood of pneumothorax and avoid chest drainage insertion after tubeless single-port VATS for pulmonary resection.
The hallmark of tubeless single-port VATS is to provide fast recovery for our surgical patients. Patients should have less pain because there is only one small incision, and no chest drain to aggravate postoperative pain (11). Patients can resume food intake and physical activity quickly because no muscle relaxant was used in the non-intubated anesthesia setting (27). Therefore, the tubeless single-port VATS is expected to fulfill the goal of fast-track surgery, by which we can allow patients to be discharged from surgery safely and quickly, also in the hope of reduced medical cost. As medical budgets become increasingly constrained in many health care systems, outpatient surgery has the potential to reduce costs. The idea of making thoracic surgery an outpatient surgery is not new. Open-lung biopsy as an outpatient procedure that was first reported by Blewett et al. in 2001 (2). Chang et al. also reported their outpatients experience with a series of patients who underwent thoracoscopic lung biopsy. In their study, the total medical cost reduction was significant in the outpatient group compared with the admission group (28). Molins et al. also reported savings for the hospital and the patients in 300 cases of outpatient thoracic surgery (29). In our study, most of the patients resumed daily activity on the day of surgery without the need for oral analgesics and all were discharged without complications. As experience with tubeless single-port VATS increases, we may push standard inpatient thoracic surgery a step forward to becoming outpatient surgery, in the hope that the cost-effectiveness of thoracic surgery can be maximized.
The limitations of this study resided on its retrospective nature. Patients were all carefully selected for accepting the tubeless single-port technique. The results should be carefully interpreted when applying to the future patients. However, this is a preliminary study focusing on the feasibility and safety of a novel surgical approach. More studies are warranted to verify the results of tubeless single-port technique.
After refining the tubeless technique with the monitoring of DDS, we believe it is a safe and scientific way to confirm the presence of air leakage after thoracoscopic lung resection. More experience will be needed to select patients appropriate for tubeless single-port VATS and to refine the maneuvers needed to avoid complications. We believe the tubeless single-port technique can make pulmonary resection a fast-track surgery, and our next goal is to reliably select the patients who will most benefit from this approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of Taipei VGH (approval number: 2017-01-016AC).
References
- Russo L, Wiechmann RJ, Magovern JA, et al. Early chest tube removal after video-assisted thoracoscopic wedge resection of the lung. Ann Thorac Surg 1998;66:1751-4. [Crossref] [PubMed]
- Blewett CJ, Bennett WF, Miller JD, et al. Open lung biopsy as an outpatient procedure. Ann Thorac Surg 2001;71:1113-5. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Liu CY, Lin CS, Liu CC. Asian perspectives in thoracic surgery: clinical innovation in Taiwan. J Thorac Dis 2016;8:S606-S612. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Han KN, Kim HK, Choi YH. Comparison of single port versus multiport thoracoscopic segmentectomy. J Thorac Dis 2016;8:S279-S286. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Watanabe A, Watanabe T, Ohsawa H, et al. Avoiding chest tube placement after video-assisted thoracoscopic wedge resection of the lung. Eur J Cardiothorac Surg 2004;25:872-6. [Crossref] [PubMed]
- Luckraz H, Rammohan KS, Phillips M, et al. Is an intercostal chest drain necessary after video-assisted thoracoscopic (VATS) lung biopsy? Ann Thorac Surg 2007;84:237-9. [Crossref] [PubMed]
- Satherley LK, Luckraz H, Rammohan KS, et al. Routine placement of an intercostal chest drain during video-assisted thoracoscopic surgical lung biopsy unnecessarily prolongs in-hospital length of stay in selected patients. Eur J Cardiothorac Surg 2009;36:737-40. [Crossref] [PubMed]
- Nakashima S, Watanabe A, Mishina T, et al. Feasibility and safety of postoperative management without chest tube placement after thoracoscopic wedge resection of the lung. Surg Today 2011;41:774-9. [Crossref] [PubMed]
- Holbek BL, Hansen HJ, Kehlet H, et al. Thoracoscopic pulmonary wedge resection without post-operative chest drain: an observational study. Gen Thorac Cardiovasc Surg 2016;64:612-7. [Crossref] [PubMed]
- Cui F, Liu J, Li S, et al. Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis 2016;8:2226-32. [Crossref] [PubMed]
- Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [PubMed]
- Yang SM, Wang ML, Hung MH, et al. Tubeless uniportal thoracoscopic wedge resection for peripheral lung nodules. Ann Thorac Surg 2017;103:462-8. [Crossref] [PubMed]
- Zheng H, Hu XF, Jiang GN, et al. Nonintubated-awake anesthesia for uniportal video-assisted thoracic surgery procedures. Thorac Surg Clin 2017;27:399-406. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-S250. [PubMed]
- Ueda K, Hayashi M, Tanaka T, et al. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardiothorac Surg 2013;44:225-9. [Crossref] [PubMed]
- Murakami J, Ueda K, Tanaka T, et al. The validation of a no-drain policy after thoracoscopic major lung resection. Ann Thorac Surg 2017;104:1005-11. [Crossref] [PubMed]
- Yamamoto S, Endo S, Minegishi K, et al. Polyglycolic acid mesh occlusion for postoperative bronchopleural fistula. Asian Cardiovasc Thorac Ann 2015;23:931-6. [Crossref] [PubMed]
- Ueda K, Tanaka T, Li TS, et al. Sutureless pneumostasis using bioabsorbable mesh and glue during major lung resection for cancer: who are the best candidates? J Thorac Cardiovasc Surg 2010;139:600-5. [Crossref] [PubMed]
- Hasegawa S, Nakayama S, Hida K, et al. Effect of tri-staple technology and slow firing on secure stapling using an endoscopic linear stapler. Dig Surg 2015;32:353-60. [Crossref] [PubMed]
- Zhang D, Miao J, Hu X, et al. A clinical study of efficacy of polyglycolic acid sleeve after video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. J Thorac Dis 2017;9:1093-9. [Crossref] [PubMed]
- Gilbert S, McGuire AL, Maghera S, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J Thorac Cardiovasc Surg 2015;150:1243-9. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Chang AC, Yee J, Orringer MB, et al. Diagnostic thoracoscopic lung biopsy: an outpatient experience. Ann Thorac Surg 2002;74:1942-6. [Crossref] [PubMed]
- Molins L, Fibla JJ, Pérez J, et al. Outpatient thoracic surgical programme in 300 patients: clinical results and economic impact. Eur J Cardiothorac Surg 2006;29:271-5. [Crossref] [PubMed]


