The role of prophylactic cranial irradiation in surgically resected combined small cell lung cancer: a retrospective study
Introduction
Combined small cell lung cancer (C-SCLC) is defined as small cell lung cancer (SCLC) combined with any of non-small cell lung cancer (NSCLC) histological types, such as large cell carcinoma, squamous cell carcinoma, or adenocarcinoma. The incidence of C-SCLC was reportedly to be 2% at diagnosis (1) and 10% after chemotherapy or radiotherapy (2) according to the data in the 1980’s. With surgery utilization increased, up to 28% of surgically resected SCLC was proved to be C-SCLC in the 2000’s (3).
Historically, the treatment of C-SCLC has always been in accordance with National Comprehensive Cancer Network Guidelines of SCLC (4), which recommend prophylactic cranial irradiation (PCI) as category 1 in limited-stage patients who attain a complete or partial response (5). For patients with extensive-stage disease, PCI is a category 2A recommendation (6). Particularly, PCI is recommended for all SCLC patients who have had a complete resection (7). The preferred dose for PCI to the whole brain is 25 Gy in 10 daily fractions. However, the guidelines did not differentiate C-SCLC from pure SCLC, while amounts of existing evidence shows that PCI provides no survival benefit when it can only reduce the incidence of brain metastases in NSCLC patients (8-10). Based on the above, whether PCI can decrease brain metastases and increase overall survival (OS) of C-SCLC is currently not well defined. To our best knowledge, optimal treatment schedule of C-SCLC has not yet been determined, and the evidence on PCI of C-SCLC patients is limited, so this study aimed to initially evaluate the effect of PCI on surgically resected C-SCLC patients in our institution retrospectively.
Methods
Eligibility criteria
All consecutive patients with pathologically diagnosed C-SCLC after surgery were identified in our hospital between January 2005 and December 2014. Pretreatment evaluation generally included physical and hematological examination, chest computed tomography (CT) scans, bronchoscopy, ultrasound examination or CT scan of abdomen, brain magnetic resonance imaging and bone radionuclide imaging. Positive emission tomography (PET)/CT was not routinely used in these patients.
The study was approved by the institutional review board/ethics committee at Shanghai Chest Hospital.
Basic information and treatment records
The clinical information of enrolled patients, including smoking history, age, gender, pathological combined components, disease extent, therapeutic strategies and survival, was obtained from the medical, radiological, and surgical records. The basic therapeutic records mainly contained the information of surgery procedures, chemotherapy, chest radiation and PCI. All included patients were restaged accordantly by the 7th edition of the American Joint Committee on Cancer tumor-node-metastasis (TNM) classification system according to the postoperative pathological examination. Surgical procedures included wedge resection, sleeve resection, lobectomy and pneumonectomy, with or without ipsilateral hilar and mediastinal lymphadenectomy. Patients received neo-adjuvant or adjuvant chemotherapy, and the chemotherapy strategies contained cisplatin or carboplatin in combination with etoposide (EP/EC), paclitaxel or docetaxel in combination with carboplatin, and vinorelbine-based or unknown cytotoxic agents.
Postoperative radiation therapy (PORT) was administered by three-dimensional conformal radiotherapy technique or intensity-modulated radiation therapy. Postoperative thoracic radiation therapy was preferred in TNM stage II–IV patients and positive-margin stage I patients, similar to the treatment principle of NSCLC. The reasons of above patients not accepting PORT were as follows: patients’ refusal, poor lung function or low Karnofsky performance status (KPS) after surgery. The clinical target volume (CTV) for left-lung cancers includes the bronchial stump and lymph node stations 2R, 2L, 3, 4R, 4L, 5, 6, 7, and 10 to 11L; and the CTV for right-lung cancers includes the bronchial stump and lymph node stations 2R, 2L, 3, 4R, 4L, 7, and 10 to 11R. The planning target volume included CTV with a 0.8- to 1-cm margin in all directions. The total dose 50–60 Gy of thoracic radiation therapy was administered with 1.8–2 Gy per fraction for 5 days a week. PCI was delivered to patients without brain metastasis after surgery and chemotherapy or chemoradiotherapy. PCI dose fractionation was 25 Gy in 10 fractions for 5 days a week. For there is no evidence of PCI in combined SCLC, so a patient should receive PCI or not is decided by the determination of radiation oncologists in our hospital and the willingness of patients.
Follow-up
Patients were generally followed every 3 months after surgery for the first 2 years and every 6 to 12 months thereafter. Regular follow-up evaluations included clinical assessments, chest CT scans, and ultrasound or CT scans of the abdomen. Treatment failure was determined based on available information, such as clinical assessments, imaging modalities and pathological diagnosis. We obtained follow-up information by conducting telephone surveys and reviewing electronic medical records in the clinic. The first treatment failure events were recorded and analyzed.
Statistical analysis
Baseline characteristics were compared using the chi-square test for categorical variables between the PCI group and non-PCI group. OS was calculated from the date of surgery to date of death from any cause or last follow-up. Disease-free survival (DFS) was calculated from surgery to the time of first recurrence, death from any cause, or last follow-up. Brain metastasis free survival (BMFS) was calculated from the date of surgery to date of imaging diagnosis of brain metastasis, death from any cause, or last follow-up. OS, DFS, and BMFS were estimated by Kaplan-Meier method, and differences in survival curves between the two groups were evaluated by log-rank test. Univariate survival analysis was performed by the Kaplan-Meier method and log-rank test. Multivariate survival analysis was performed by a Cox proportional hazards model and a backward-forward stepwise method was selected. Two-sided P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS software (version 22.0; IBM-SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
Between January 2005 and December 2014, 105 patients were diagnosed with C-SCLC by surgery, bronchoscopy or percutaneous aspiration lung biopsy, and 101 patients who received surgery were qualified, however, 10 of the 101 patients were unable to follow up because of losing contact. Ultimately 91 patients were enrolled in the analysis, including nine patients with positive surgical margin. On account of different therapy modalities, 11 (12.1%) patients were in PCI group and 80 (87.9%) in non-PCI group.
There was no difference in the frequency distribution of variables between the two groups except that PCI group included a higher proportion of patients received PORT. Patient characteristics of the two groups are summarized in Table 1. Of the total 91 patients, there were 84 (92.3%) men and 7 (7.7%) women. The median age at the time of operation was 61 years. In addition to the basic features mentioned in the table, it should be specifically illustrated that two patients (2.2%) of non-PCI group were post-operatively pathologically-confirmed to be parietal pleural invasion or diaphragm invasion. The most common combined component was large cell neuroendocrine carcinoma (LCNEC, N=43, 47.3%), followed by squamous cell carcinoma (N=21, 23.0%), unspecified carcinoma component (N=15, 16.5%), adenocarcinoma (N=10, 11.0%), poorly differentiated carcinoma component (N=1, 1.1%), and giant cell and spindle cell carcinoma (N=1, 1.1%). In both groups, there was one patient receiving lobectomy plus wedge resection respectively, besides, one patient of non-PCI group received bi-lobectomy. Almost all patients received neo-adjuvant or adjuvant chemotherapy, and the most common strategies among these patients were EP/EC (N=79, 86.8%), followed by paclitaxel or docetaxel in combination with carboplatin (N=4, 4.4%), the rest may receive vinorelbine-based chemotherapy (N=2, 2.2%), and etoposide alone (N=1, 1.1%). Besides, there were four patients receiving unknown cytotoxic agents (N=4, 4.4%) and one patient received no chemotherapy (N=1, 1.1%).
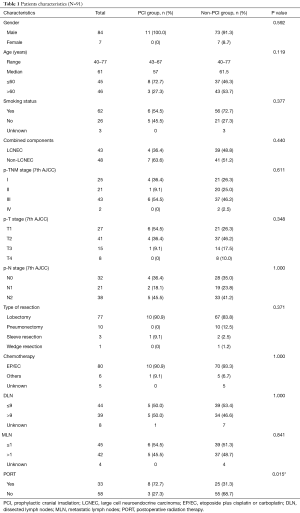
Full table
Of thirteen patients who received neo-adjuvant chemotherapy, five patients (38.5%) reached a lower T-stage and N-stage after neo-adjuvant chemotherapy, two patients (15.4%) reached a lower T-stage alone and another two patients (15.4%) only reached a lower N-stage. The failure pattern of non-PCI group occurred most in brain (N=16, 20.0%), followed by local-region (N=12, 15.0%), bone (N=10, 12.5%), liver (N=5, 6.3%), pleura (N=3, 3.8%), adrenal gland (N=3, 3.8%), lung (N=1, 1.2%) and subcutaneous nodule (N=1, 1.2%), while the only failure pattern of PCI group occurred in local-region (N=2, 18.2%).
Survival analysis and principal patterns of failure
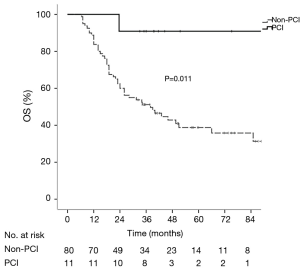
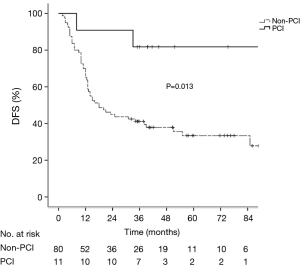
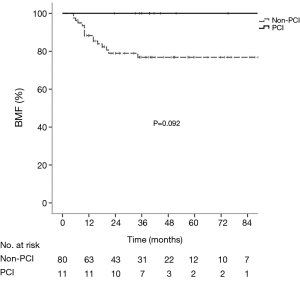
The median follow-up period of the whole group was 35.1 months (range, 6.5–113.6 months). The median OS, DFS and BMFS were 43.0, 38.1 months and not-reached, respectively. The 1-, 2- and 5-year OS rates were 90.1%, 63.7% and 44.1% respectively, the corresponding DFS rates were 68.1%, 50.5%, and 38.8%, and the corresponding BMFS rates were 89.8%, 81.8%, and 80.1%. The 5-year cumulative incidence of brain metastasis was 22.2% in the whole group. Patients in the PCI group had significantly longer OS (χ2=6.54, P=0.011) and DFS (χ2=6.23, P=0.013) than those in the non-PCI group (Figures 1,2), and had the trend to live a longer BMFS with no statistical significance (χ2=2.83, P=0.092) (Figure 3). The 1-, 2-, and 5-year OS rates of PCI group were 100.0%, 90.9% and 90.9%, while the corresponding rates of non-PCI group were 83.8%, 60.0% and 38.8%. The median OS in PCI and non-PCI group were not-reached versus 38.0 months. The 1-, 2-, and 5-year DFS rates of PCI group were 90.9%, 90.9% and 81.8%, while the corresponding rates of non-PCI group were 65.0%, 45.0% and 33.5%. The median DFS in PCI and non-PCI group were not-reached versus 18.0 months. The 1-, 2-, and 5-year BMFS rates of PCI group were 100.0%, 100.0% and 100.0% respectively, while the corresponding rates of non-PCI group were 88.3%, 78.9% and 76.9%. The median BMFS of two groups was not reached. Additionally, the leading failure pattern of non-PCI group and the whole group was brain metastasis, and the 5-year cumulative incidence of brain metastasis reached up to 26.3% in the non-PCI group.
Subgroup analyses
Results of the subgroup analyses by a Cox proportional hazards model are shown in Table 2. PCI significantly improved the OS of patients with receiving lobectomy, EP/EC chemotherapy and PORT, respectively. For the patients receiving lobectomy, the 1-, 2-, and 5-year OS rates of PCI group were 100.0%, 90.0% and 90.0%, while the corresponding rates of non-PCI group were 85.1%, 61.2% and 37.6% [hazard ratio (HR) =0.127, P=0.042]. For the patients receiving EP/EC chemotherapy, the 1-, 2-, and 5-year OS rates of PCI group were 100.0%, 90.0% and 90.0%, while the corresponding rates of non-PCI group were 83.8%, 58.8% and 36.2% (HR =0.127, P=0.042). For the patients receiving PORT, the 1-, 2-, and 5-year OS rates of PCI group were 100.0%, 87.5% and 87.5%, while the corresponding rates of non-PCI group were 76.0%, 48.0% and 24.0% (HR =0.110, P=0.032). For the patients with T1-2 stage, N+ disease, or those with pathologically confirmed combined squamous cell carcinoma, unspecified carcinoma, adenocarcinoma, poorly differentiated carcinoma, and giant cell and spindle cell carcinoma, PCI group had the trend to live a longer OS with marginal significance (P=0.053, P=0.069 and P=0.050, respectively).

Full table
Univariate analysis and multivariate analysis
The patient characteristics were evaluated to determine their prognostic value for OS in Table 3. Univariate survival analysis showed that PCI (χ2=6.54, P=0.011), smoking status (χ2=13.18, P<0.001), combined components (χ2=6.14, P=0.013) and p-TNM stage (χ2=9.66, P=0.022) were significant prognostic factors for OS. Multivariate analysis revealed that PCI (HR =0.102, P=0.024), tobacco smoking (HR =3.454, P=0.001) and pathologically combined LCNEC (HR =0.479, P=0.015) were independent prognostic factors of the OS.
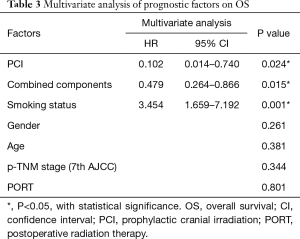
Full table
Discussion
The clinical outcomes of C-SCLC among various studies disaccord, median OS ranged from 9.4 to 27 months for TNM stage I–IV C-SCLC patients, 1-year OS ranged from 38.6% to 76.6%, and median PFS ranged from 10.5 to 13.1 months (11-14). The median OS ranged from 20 to 62.2 months for surgically resected C-SCLC patients, 1-year OS ranged from 76.6% to 86.6%, 5-year OS ranged from 42.3% to 50.2%, and median DFS ranged from 14.5 to 43.4 months (15,16). Accordingly, the median OS and DFS in our analysis were 43.0 and 38.1 months in postoperative stage I–IV C-SCLC, and the 1- and 5-year OS rates in our analysis were 90.1% and 44.1%. As a result, C-SCLC patients in our study were consecutively treated as a way to minimize bias.
In our retrospective analysis, receipt of PCI in patients with pathologically diagnosed C-SCLC after surgery, was demonstrated to be associated with improved OS and DFS. Furthermore, patients of PCI group had the trend to live a longer BMFS dramatically, though the difference was not statistically significant. Considering the small sample size in our analysis, the dramatical statistical significance can be reached (χ2=5.70, P=0.017) when doubling the sample size of the two groups, which demonstrate that bigger sample size may lead to significant results. The baseline characteristics compared by the chi-square test showed the statistical difference in the frequency distribution of patients receiving PORT, however, the influence on OS of PORT should be eliminated for its no significance when doing the univariate and multivariate survival analysis. In the subgroup analyses, PCI significantly improved OS of patients with receiving lobectomy, EP/EC chemotherapy or PORT, which may provide scientific guidance for optimal comprehensive treatment modalities in C-SCLC, like uniting lobectomy, EP/EC chemotherapy or PORT, with PCI. The value of PCI in patients with T1–2 stage, N+ disease, or combined non-LCNEC, should be verified with much bigger sample size in following studies. Although the univariate analysis showed p-TNM stage was one of prognostic factors for OS, multivariate analysis failed to authenticate its prognostic value. The analysis revealed that PCI and pathologically combined LCNEC were protective factors for patients with pathologically diagnosed C-SCLC after surgery, and HR value of PCI (HR =0.102) reminded us of its strong protection, while tobacco smoking was the sole risk factor (HR =3.454). Apparently, the significance of p-TNM stage and the protective role of combined LCNEC in this C-SCLC survival analysis were discordant with previous research, and the critical factors should be the prolongation of follow-up period and the expanding time scope of enrollment in our analysis.
Although the evidence of brain metastasis and the importance of PCI in C-SCLC patients were rare, the scant data sufficiently remind the positive role of PCI for decreasing brain metastases and increasing OS of combined SCLC. For lack of research on failure patterns of C-SCLC, only three studies elaborated that brain metastasis rates ranged from 12.3% to 22.2% (2,11,12), when mentioning the leading failure patterns were brain metastasis, lung metastasis, bone metastasis and liver metastasis in order. Besides, we reviewed the data of brain metastasis incidence in SCLC and NSCLC, Wu et al. (17) demonstrated that the 5-year cumulative incidence of brain metastasis for stage I/II and III SCLC were 12% and 26%, while the meta-analysis of randomized controlled trials of PCI in NSCLC (18) showed the incidence of brain metastases in no-PCI group was 18.6% (122/657). In our analysis, brain metastasis was the most common failure pattern in the non-PCI group, while no brain metastasis happened in the PCI group, and 5-year cumulative incidence of brain metastasis reached up to 22.0% and 23.6% for stage I/II and stage III–IV C-SCLC. In addition, the log-rank test showed that p-TNM stage (χ2=36.43, P<0.001) was significant prognostic factor for BMFS, and Cox proportional hazards model revealed that p-stage IV was independent risk factor of BMFS when compared to p-stage I (HR =34.972, P<0.001), while p-stage II and p-stage III were not (HR =1.294, P=0.716; and HR =1.002, P=0.998). Consequently, the cumulative incidence of brain metastasis for surgically resected C-SCLC approached to stage II-III SCLC or a little more than NSCLC. Except for surgery in stage I SCLC, a few recent reports have demonstrated excellent outcomes of stereotactic body radiation therapy for stage I SCLC, which showed PCI should be recommended for pathologic stage I SCLC because relative high brain-metastases rates have also been observed, from 7.5% to 14.3%, while none of the 17 patients (0%) who had undergone PCI developed brain metastases (19-21). Though there is no research of PCI on C-SCLC, based on current data of PCI on early-stage SCLC, and the contribution of PCI to improving OS and DFS in our study, taken together, it may call for the utilization of PCI in C-SCLC patients treated by surgery, to a certain extent.
Above all, patients treated with PCI had a lower incidence of brain metastasis, though the p-value was close to but not less than 0.05. The reasonable explanation was the small sample size and just a-single institutional experience. This study demonstrated that PCI could contribute to improvement of OS and DFS, which may be an indication of its profound value in C-SCLC. In combination with the fact that PCI can decrease brain metastases and increase OS in limited-stage SCLC, we put forward the hypothesis that PCI was indispensable in the treatment of C-SCLC, however, it would need to be further verified.
There were several limitations in this study. Firstly, the analysis was retrospective and inevitable biases may affect the conclusions. The factor unevenly distributed between the two groups was difficult to balance, though it was not an influence-on-survival factor. Secondly, our study could not reach the statistical significance of BMFS due to the small sample size, though patients of PCI group had the significant trend to live a longer BMFS. Thirdly, the study could not bring all risk factors or protective factors into the analysis, for the unobtainable information of new risk factors discovered by recent research, such as tumor mutation burden, microsatellites instability, lncRNA and platelets-related factors, etc. Finally, for lack of detailed information about the influence of PCI on quality of life for all patients, the professional assessment of potential toxicity could not be acquired. Consequently, whether PCI can decrease brain metastases and increase OS of combined SCLC should be further explored in prospective randomized trials.
Conclusions
Combined SCLC patients have a relative high risk of developing brain metastases based on our study. These data showed that PCI could significantly improve the OS and DFS of surgically resected C-SCLC patients. Patients in PCI group had the trend to live a longer BMFS. The role of PCI in patients with C-SCLC should be further investigated in prospective randomized trials.
Acknowledgements
Funding: This study was supported by the Innovation Fund for PhD Students from Shanghai Jiao Tong University School of Medicine (BXJ201742).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board/ethics committee at Shanghai Chest Hospital.
References
- Adelstein DJ, Tomashefski JF Jr, Snow NJ, et al. Mixed small cell and non-small cell lung cancer. Chest 1986;89:699-704. [Crossref] [PubMed]
- Mangum MD, Greco FA, Hainsworth JD, et al. Combined small-cell and non-small-cell lung cancer. J Clin Oncol 1989;7:607-12. [Crossref] [PubMed]
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [Crossref] [PubMed]
- NCCN. Non-Small Cell Lung Cancer (Version 2. 2018) 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Sun DS, Hu LK, Cai Y, et al. A systematic review of risk factors for brain metastases and value of prophylactic cranial irradiation in non-small cell lung cancer. Asian Pac J Cancer Prev 2014;15:1233-9. [Crossref] [PubMed]
- Le Péchoux C, Al Mohkles H, Dhermain F. Present role of prophylactic cranial irradiation. Bull Cancer 2013;100:35-43. [PubMed]
- Xie SS, Li M, Zhou CC, et al. Prophylactic cranial irradiation may impose a detrimental effect on overall survival of patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9. [Crossref] [PubMed]
- Kim YH, Mishima M, Date H. "Combined" small cell and "pure" small cell lung cancer: is there a clinical difference? Med Oncol 2013;30:600. [Crossref] [PubMed]
- Moniodis A, Racila E, Divo M. Case report: combined small cell lung cancer in a lung transplant recipient. Transplant Proc 2015;47:852-4. [Crossref] [PubMed]
- Zhang C, Yang H, Zhao H, et al. Clinical outcomes of surgically resected combined small cell lung cancer: a two-institutional experience. J Thorac Dis 2017;9:151-8. [Crossref] [PubMed]
- Wallace AS, Arya M, Frazier SR, et al. Combined small-cell lung carcinoma: An institutional experience. Thorac Cancer 2014;5:57-62. [Crossref] [PubMed]
- Men Y, Luo Y, Zhai Y, et al. The role of postoperative radiotherapy (PORT) in combined small cell lung cancer (C-SCLC). Oncotarget 2017;8:48922-9. [Crossref] [PubMed]
- Shao N, Cai Q. High pretreatment neutrophil-lymphocyte ratio predicts recurrence and poor prognosis for combined small cell lung cancer. Clin Transl Oncol 2015;17:772-8. [Crossref] [PubMed]
- Wu AJ, Gillis A, Foster A, et al. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother Oncol 2017;125:130-5. [Crossref] [PubMed]
- Al Feghali KA, Ballout RA, Khamis AM, et al. Prophylactic Cranial Irradiation in Patients With Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Oncol 2018;8:115. [Crossref] [PubMed]
- Videtic GM, Stephans KL, Woody NM, et al. Stereotactic body radiation therapy-based treatment model for stage I medically inoperable small cell lung cancer. Pract Radiat Oncol 2013;3:301-6. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Allen PK, et al. Outcomes of Stereotactic Body Radiotherapy for T1-T2N0 Small Cell Carcinoma According to Addition of Chemotherapy and Prophylactic Cranial Irradiation: A Multicenter Analysis. Clin Lung Cancer 2017;18:675-81.e1. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Allen PK, et al. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:362-71. [Crossref] [PubMed]


