Radiopaque preventive landmarks’ placement during stentless bioprosthesis implantation
Introduction
In patients with aortic stenosis, bioprosthetic valves are increasingly used. Their main advantage is that they do not require constant anticoagulation therapy, due to their lower thrombotic risk compared with mechanical valves, so patients with bioprosthetic valves have a significantly diminished risk of haemorrhage (1,2). Although their benefits, they are also presenting limitations, as their time-related degeneration (3,4). Reoperation which was, until a few years ago, the only treatment for this condition, carries a significant surgical risk, especially in patients with multiple comorbidities (5), so the benefit of less invasive technique enabling the implantation of aortic valve prosthesis [transcatheter aortic valve-in-surgical aortic valve (TAV-in-SAV)] by a percutaneous access is remarkably important (6). Patients eligibility is decided by a heart team, and imaging plays a key role in this selection, focusing on correct identification of bioprosthetic aortic valves type and size, and recognition of patients at increased anatomical risk for coronary artery occlusion (6,7). Radiolucency of stentless bioprosthetic valves, represent a significant challenge.
Methods
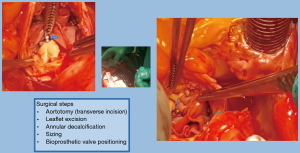
We propose a new surgical technique with the addition of landmarks using a knot-tying device which consist of radiopaque Titan clips (Cor-Knot device, LSI Solutions, Inc., Victor, NY, USA) for marking the new annulus. The surgical aortic valve replacement (SAVR) by bioprosthesis was performed using a stentless valve with no radiopaque components (Freedom Solo, Sorin). This valve is created by two bovine pericardial sheets without texture reinforcement. The design pursues the natural shape of native aortic annulus and commissures. This allows a less complicated technique of implantation in a strictly supra-annular position with a single suture line. The implantation starts with three 4-0 polypropylene sutures placed in a supra-annular position at the midpoint of each sinus and then passed through the external pericardial flange of the valve. The bioprosthetic valve is then parachuted into the aortic root and tied. Consequently, these stitches are placed 2 mm above the annulus, in a continuity (8). At the level of the commissures, each suture is passed out of the aorta and tied (with or without pledget) with the suture coming from the adjacent sinus (9-11) (Figure 1). The supra-annular implantation provides a greater effective orifice area index for a given valve size. The strictly supra-annular create a new annulus which is difficult be identified.
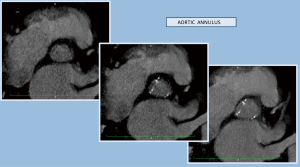
The chosen imaging method, in order to evaluate the results of the operation, was computed tomography (CT) scanning (64-slice MDCT, Brilliance, Philips). The study consisted of a thin sliced contrast electrocardiograph (ECG) gated chest CT (1 systolic-1 diastolic cardiac phase), trying to simulate the required assessment of aortic root the evaluation of radiopaque placed markers (6,7,12-17) (Figures 2-4).
As there was no randomization, no new treatment being explored and no potential harm to the patients there was no need for ethical approval by the ethics committee of “Evangelismos” General Hospital of Athens, Greece. However, researchers received patient consent after being informed about the type, the purpose of the study and the right to refuse to participate or to withdraw consent to participate any time without reprisal.
Results
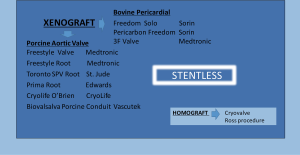
SAVR with a bioprosthesis can be performed using either a stented or a stentless or a sutureless valve (3,6,7,13,14).
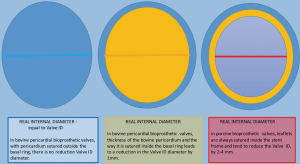
Stented valves are most of the time constructed using a radiopaque base ring stent frame from which three stent posts arises at a right angle to support the valve leaflets, which serve as perfect markers for positioning of transcatheter valves. There are constructed from porcine or bovine pericardial tissue (Figure 5).
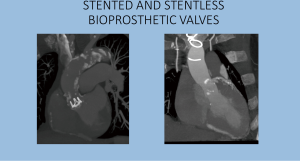
Sutureless valves are self-expandable frame valves, maintained in situ by the radial force of its stent, no sutures required. Stentless valves are most often constructed from porcine or bovine pericardial tissue or human aortic root tissue, lack a rigid scaffold, and have no radiopaque components (Figures 5,6).
In patients with aortic stenosis, bioprosthetic valves are increasingly used while they are often preferred over mechanical valves, as they do not need long-term anticoagulation with associated risks of bleeding and thromboembolism (1,3). Many surgeons are preferring using stentless valves, as they offer an easier and smarter implantable technique, providing superior hemodynamic performance due to the absence of a suture stent-ring and therefore no obstructions to blood flow.
But bioprostheses are also presenting limitations, and the most important one, is their time-related degeneration and structural failure, potentially resulting in either valve stenosis, or regurgitation or combination of both (3,4). This dysfunction can be the result of calcifications or pannus formation, thrombosis, leaflet wear and tear, and endocarditis (6). However, a “redo” surgery can be associated with substantial mortality and morbidity, particularly in elderly patients and those with significant comorbidities. A transcatheter aortic valve implantation (TAVI) in a SAVR has emerged as a less-invasive alternative (TAV-in-SAV) to conventional redo surgery for bioprosthetic valve dysfunction (3,12).
Although several technical difficulties have been associated with TAV-in-SAV in a degenerate stentless bioprostheses, notably the absence of radiopaque landmarks from a stent frame or sewing ring and the use of various implantation techniques among surgical operators, presenting the greatest challenge as it may impact the relationship of the prosthetic annulus to the coronary ostia (18), increasing the risk of complications such as coronary obstruction device migration and embolization (13). In order to overcome the challenges in correct TAVI deployment in stentless valves, careful steps with contrast injection and slow deployment or some guide wire tricks such as positioning of a wire in left main coronary artery (14) or a mainly guided by transoesophageal echocardiography procedure, have been proposed.
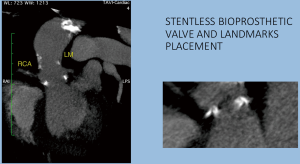
The preventive implantation of metallic vascular clips marking the aortic annulus during the operation in order to have some useful radiopaque landmarks, is a great assistance promoting better orientation and correct identification of the position of the bioprosthetic valve. The postoperative computed tomographic scan depicted the annular plane by the Titan clips. It was sufficient to mark it by placing three clips additional to the running suture at evenly distributed locations (Figures 7-9).

Conclusions
We are suggesting the preventive implantation of radiopaque landmarks, during SAVRs, using tissue valves which are lacking fixed anatomic markers, as a guide for a presumptive TAV-in-SAV procedure, keeping in mind that appropriate imaging guidance is crucial and can prevent valve misplacement, coronary obstruction and other potentially lethal complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As there was no randomization, no new treatment being explored and no potential harm to the patients there was no need for ethical approval by the ethics committee of “Evangelismos” General Hospital of Athens, Greece. However, researchers received patient consent after being informed about the type, the purpose of the study and the right to refuse to participate or to withdraw consent to participate any time without reprisal.
References
- Webb JG, Dvir D. Transcatheter aortic valve replacement for bioprosthetic aortic valve failure: the valve-in-valve procedure. Circulation 2013;127:2542-50. [Crossref] [PubMed]
- Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence 2011;5:91-9. [Crossref] [PubMed]
- Bapat V, Mydin I, Chadalavada S, et al. A guide to fluoroscopic identification and design of bioprosthetic valves:a reference for valve-in-valve procedure. Catheter Cardiovasc Interv 2013;81:853-61. [Crossref] [PubMed]
- Blanke P, Soon J, Dvir D, et al. Computed Tomography Assessment for Transcatheter Aortic Valve in Valve Implantation: The Vancouver Approach to Predict Anatomical Risk for Coronary Obstruction and Other Considerations. J Cardiovasc Comput Tomogr 2016;10:491-9. [Crossref] [PubMed]
- Jones JM, O'kane H, Gladstone DJ, et al. Repeat heart valve surgery: risk factors for operative mortality. J Thorac Cardiovasc Surg 2001;122:913-8. [Crossref] [PubMed]
- Piazza N, Bleiziffer S, Brockmann G, et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve: from concept to clinical application and evaluation (part 1). JACC Cardiovasc Interv 2011;4:721-32. [Crossref] [PubMed]
- Lama N, Maniatis P, Patris V, et al. TAV in SAV: what “heart team” needs to know. Poster: ECR, 2017.
- Aymard T, Eckstein F, Englberger L, et al. The Sorin Freedom SOLO stentless aortic valve:technique of implantation and operative results in 109 patients. J Thorac Cardiovasc Surg 2010;139:775-7. [Crossref] [PubMed]
- Czerny M, Sündermann S, Falk V. The Cor-Knot device may serve as an ideal radiopaque marker of the annular plane for future valve-in-valve implantation. Ann Thorac Surg 2014;98:1485-6. [Crossref] [PubMed]
- Di Eusanio M, Saia F, Pellicciari G, et al. In the era of the valve-in-valve: is transcatheter aortic valve implantation (TAVI) in sutureless valves feasible? Ann Cardiothorac Surg 2015;4:214-7. [PubMed]
- Brescia AA, Bolling SF, Patel HJ. Valvular Regurgitation After Implantation of Prostheses Secured with Cor-Knot Automated Fasteners. Ann Thorac Surg 2017;103:e491-2. [Crossref] [PubMed]
- Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc Interv 2014;7:115-27. [Crossref] [PubMed]
- Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [Crossref] [PubMed]
- Valve in Valve. Collaboration between the technology company UBQO and Dr. Vinayak Bapat, Consultant Cardiac Surgeon at St. Thomas Hospital, London, UK. Available online: http://www.ubqo.com/viv
- Schoenhagen P, Hausleiter J, Achenbach S, et al. Computed tomography in the evaluation for transcatheter aortic valve implantation (TAVI). Cardiovasc Diagn Ther 2011;1:44-56. [PubMed]
- Leipsic J, Gurvitch R, Labounty TM, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011;4:416-29. [Crossref] [PubMed]
- Erlebach M, Wottke M, Deutsch MA, et al. Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprosthetic valves: consecutive patients in a single-center setting. J Thorac Dis 2015;7:1494-500. [PubMed]
- Duncan A, Davies S, Di Mario C, et al. Valve-in-valve transcatheter aortic valve implantation for failing surgical aortic stentless bioprosthetic valves: A single-center experience. J Thorac Cardiovasc Surg 2015;150:91-8. [Crossref] [PubMed]