Does protruding type 1 esophageal cancer really have a good response to radiation therapy?—a retrospective observational study
Introduction
Polypoid or exophytic carcinoma of the esophagus has long been reported to have a better outcome with surgery and/or radiation therapy (RT) than other types of esophageal cancer. However, these studies were conducted in the 1980s and 1990s, when responses to RT in “proliferative” or “tumorous” disease types were evaluated by esophagography (1-3). With recent advances in endoscopic technology, accurate tumor typing is commonly achieved on the basis of macroscopic endoscopic findings (4). RT is frequently used to treat locally advanced esophageal cancer; however, no studies have evaluated the association between responses to RT and type of esophageal cancer as determined endoscopically. In this retrospective study, we therefore compared responses to RT in patients with locally advanced esophageal cancer according to the macroscopic disease type determined on the basis of endoscopic findings. We also assessed responses to RT in patients who had undergone at least two follow-up endoscopies.
Methods
Patients
Table 1 lists relevant patients’ characteristics and their treatment regimens.
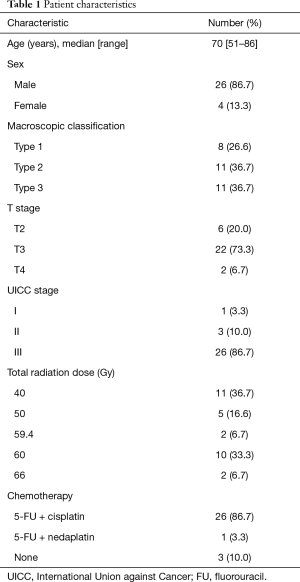
Full table
Data on 30 patients (26 men and 4 women) with endoscopic and pathological diagnoses of squamous cell carcinoma (SCC) of the thoracic esophagus who had not undergone surgery after RT were reviewed. All patients had undergone RT to the primary lesion and lymph node regions from January 2012 to November 2017 at our hospital. All tumor samples had been collected before treatment. Patient age at RT initiation ranged from 51 to 86 years (median, 70 years). Clinical stage was assessed according to the TNM classification [the International Union Against Cancer (UICC), 2009; stages IB/IIA/IIB/IIIA/IIIB/IIIC =1/1/2/7/12/7, respectively]. This retrospective study was approved by the Institutional Review Board of Nihon University School of Medicine. The patients had provided informed consent for all treatment procedures.
Macroscopic classification of esophageal cancer
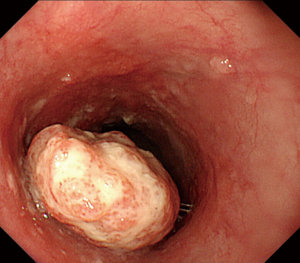
In accordance with the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus of the Japanese Society for Esophageal Disease (11th edition) (4), advanced disease was classified on the basis of endoscopic findings as one of the following types: (I) protruding; (II) ulcerative and localized; (III) ulcerative and infiltrative; (IV) diffusely infiltrative; and (V) unclassified. Eight patients had type 1 disease, 11 type 2, 11 type 3, none type 4, and none type 5. Type 1 lesions are tall and protruding with an eroded surface but no ulceration; they correspond with polypoid or exophytic type tumors on esophagography images (Figure 1). In this study, type 1 lesions were diagnosed by pathological examination as moderately differentiated (M/D) SCC in seven patients and well differentiated (W/D) in one. Other types were diagnosed as M/D in 11 patients, W/D in two, poorly differentiated in one, and only SCC in eight. There was no difference in degree of differentiation between type 1 and other types of tumor.
RT
A linear accelerator was used to deliver beam energy of 10 MV to all patients except one, who received beam energy of 4 MV. Multiple fields were used with an anterior-posterior opposed field that included at least the primary tumor, lymph nodes harboring metastases and, when appropriate, regional lymph nodes. The total dose ranged from 40 to 66 Gy (median, 50 Gy) and was 40 Gy in patients who either did not consent to post-chemoradiation surgery or had a good response to RT; these patients were followed closely. Two patients who had undergone RT alone each received a total dose of 66 Gy. The dose per fraction was 2 Gy except in two patients with a wide radiation field, for whom 1.8 Gy was used.
Chemotherapy
Twenty-seven of the 30 patients received concurrent chemotherapy. The regimen used was 5-fluorouracil (5-FU) + cisplatin [cis-diamine dichloroplatinum (CDDP)] in 26 patients and 5-FU + nedaplatin [cis-Diammine(glycolato-O1,O2)platinum (CDGP)] in one. All patients except one completed the 5-FU + CDDP regimen specified by the Japan Clinical Oncology Group Trial (JCOG9516) schedule. Three patients underwent RT alone because of advanced age and renal impairment.
Response to RT
The response of the primary lesions was assessed on the basis of endoscopic findings approximately 1 month after completion of RT; the first endoscopic examination being performed 38 to 174 days after initiation of RT (median, 74 days). These responses were evaluated according to the criteria for endoscopic complete response (CR) of primary lesions in the Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus of the Japanese Society for Esophageal Disease (11th edition) (5,6). CR was diagnosed when all of the following criteria were met: (I) resolution of all endoscopic findings suggesting the presence of a tumor; (II) negative endoscopic biopsy findings in the area of the primary tumor; (III) entire esophagus observed by endoscopy; and (IV) no endoscopic evidence of active esophagitis. Lesions that appeared to be residual tumors that had diminished in size, biopsies of which showed no malignant cells, and with no tumor growth noted at the second and subsequent follow-up endoscopic examinations were also diagnosed as achieving CR. Responses of the primary lesions were assessed at the last endoscopic examination (performed 71 to 779 days after initiation of RT; median, 397 days) in the 18 patients who had undergone follow-up endoscopy at least twice.
Statistical methods
SPSS ver. 21.0 (IBM, Armonk NY, USA) was used for statistical analysis. Univariate analysis using Pearson’s χ2 test and multivariate analysis using stepwise logistic regression were performed to analyze primary responses to RT. The following patient characteristics were evaluated: age (< median 70 vs. ≥70 years), clinical T staging (T2 vs. T3–4), macroscopic findings on endoscopy (type 1 vs. other types), and radiation dose (< median 50 vs. ≥50 Gy). The Kaplan-Meier method was used to calculate the probability of disease-specific survival (DSS) from the date of RT initiation. Differences in survival between subgroups of patients according to clinical stage (IB/IIA/IIB/IIIA vs. IIIB/IIIC) and the above variables were analyzed using Mantel’s log-rank test.
Results
Comparisons between patients with type 1 disease and those with other disease types according to primary response to RT are shown in Table 2. The χ2 test revealed that patients with type 1 disease had a significantly higher CR rate than those with other types (P=0.041). Univariate analysis showed no significant differences for the other variables.

Full table
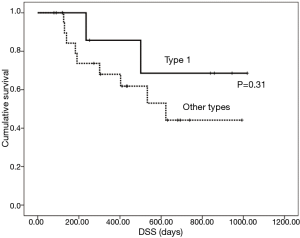
The results of multivariate analysis aimed at determining correlations between the studied factors and CR of the primary tumor are shown in Table 3. According to stepwise logistic regression analysis, only macroscopic classification type 1 showed a higher CR rate than other types (P=0.05). Changes in primary tumor response between the first and last endoscopy in the 18 patients who had undergone at least two follow-up endoscopies are shown in Table 4. After the first endoscopy, additional therapy with oral tegafur-gimestat-otastat potassium (TS-1) was administered to 11 patients and paclitaxel (PTX) to one. Six patients received no additional therapy. According to the χ2 test, patients with type 1 disease had a significantly higher CR rate than those with other types (P=0.019) at the time of the last endoscopy. The mean DSS was 684 days for all patients; DSS did not differ between patients with type 1 disease and those with other types (P=0.31; Figure 2). Patients with clinical T2 disease and ≤ stage IIIA showed better outcomes than those with other stages (P=0.041 and 0.025, respectively).
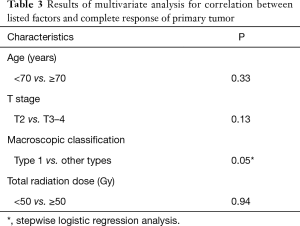
Full table

Full table
Discussion
Polypoid carcinoma of the esophagus is relatively rare, reportedly accounting for 2% to 8% of esophageal tumors and 7% of definite SCCs of the esophagus (7-9). In our study, 8 of 30 patients (26.6%) had type 1 disease, which is a higher percentage than that reported in previous studies. The percentage may have been lower if we had also enrolled patients who had undergone surgery. It has long been reported that clinical and histopathological features differ between type 1 disease and the other types. Type 1 disease also reportedly has better therapeutic outcomes than the other types. A meta-analysis has demonstrated that preoperative chemoradiotherapy (CRT) improves the prognosis of locally advanced esophageal cancer (10). Another study has shown that definitive CRT improves the prognosis of unresectable locally advanced esophageal cancer; these findings have led to increased use of RT in such patients (11). In the present study, we evaluated primary responses to RT in patients who had endoscopic macroscopic diagnoses of protruding disease type; endoscopy provides more accurate diagnoses than esophagography. To the best of our knowledge, this is the first report on responses to RT in patients with endoscopically diagnosed type 1 polypoid carcinoma of the esophagus. Only one published study has reported associations between macroscopic type of primary lesion and responses to RT. In that study, four of 15 patients had type 1 disease, three of whom achieved CR at the primary site following definitive CRT; however, responses were not evaluated according to disease type in that study (12). In our study, patients with macroscopic type 1 disease on endoscopy had better primary responses to RT than those with other types. The results of our study, in which we classified lesions macroscopically on the basis of endoscopic findings, thus supported the findings of previous studies that used esophagography findings. Of the 18 patients who had undergone follow-up endoscopy at least twice, four with type 1 disease and six with other disease types had received RT at a total dose of 40 Gy, which is lower than the recommended dose of 50.4–60 Gy for esophageal cancer (5,13). Interestingly, in those 10 patients, CR of the primary tumor (i.e., a good response to RT even at a lower than recommended total dose) was diagnosed on the last endoscopy in three of the four patients with type 1 disease (75%), whereas it was only diagnosed in one of the six patients with the other disease types (16.7%). These findings suggest that type 1 polypoid carcinoma of the esophagus is more susceptible to RT than the other types.
We have identified three studies on histopathological findings that may explain the better response of type 1 disease than of the other types to RT. One study on histopathological features of resected specimens of polypoid carcinoma of the esophagus reported that their depth of invasion was shallow (14). In another study that evaluated features of polypoid SCC in resected specimens, the incidence of adventitial involvement was lower in the polypoid type than in the other types (9). A third study used immunostaining with an anti-CD34 antibody (found in vascular endothelial cells) to compare the number of blood vessels in resected specimens of SCCs of the esophagus (15). There tended to be more numerous blood vessels per unit area in type 1 carcinoma than in the other types. The higher density of blood vessels may result in minimal numbers of hypoxic tumor cells, resulting in greater susceptibility to RT. Moreover, type 1 SCCs diagnosed endoscopically as having a white protruding portion are reportedly less common and have narrower bases and less frequent T3 adventitial involvement than those with a red protruding portion (16). In our study, the lesions of only two of eight patients with type 1 disease had a white protruding portion and no evidence of adventitial involvement on computed tomography images. Given that we examined endoscopically-obtained biopsies rather than resected specimens, it is possible that the biopsied parts were not always representative of all of the tumor. For example, esophageal carcinosarcoma (ES) is commonly polypoid. One study reported that, although polypoid ES tumors are mostly sarcomatous, their bases are characteristically mainly SCC (17). Hence, biopsy of the base of a type 1 ES tumor may result in a diagnosis of SCC. ES is more likely to be localized than other pathological tumor types and the 5-year overall survival rate is significantly better than that of esophageal carcinoma (18). Another tumor type in which biopsy specimens may be unrepresentative is esophageal basaloid SCC (EBSCC), which is characterized by submucosal tumor-like growth and can form a polypoid tumor. Hence, biopsy of the superficial portion of such a type 1 tumor may result in a diagnosis of SCC (19). We have previously reported a case of EBSCC with a good response to RT (20).
We assessed the responses of primary lesions to RT endoscopically at a median of 74 days after initiation of RT; however, this may have been too early. The single published study on the optimal timing of endoscopic evaluation of esophageal cancer after definitive CRT found that the mean time to CR at the primary site was 97 days after initiation of CRT/RT. In that study, biopsy specimens showed residual viable cancer cells within 75 days of CRT/RT initiation in four patients, these cells not being identified in subsequent biopsies (21). These authors recommended that tumor response should be evaluated endoscopically 75 to 90 days after initiation of CRT/RT. Because most patients in our institution were receiving preoperative RT, responses to RT had to be assessed earlier than this, which is why we evaluated responses to RT only in those study patients who had undergone follow-up endoscopy at least twice; at a median of 397 days after initiation of RT, the response of type 1 tumors to RT was significantly better than that of the other types and better than that at the time of the first endoscopy. In contrast, type 1 disease and the other types did not differ significantly in DSS rate, which may be partly because a radiation dose of 40 Gy is too low to treat lymph node metastases, the optimal dose being 50.4–60 Gy (5,13).
Conclusions
In future studies, we plan to evaluate responses to RT according to macroscopic disease type by histopathological examination of specimens resected after RT in a larger cohort of patients receiving radical dose RT. We will also further explore findings and optimal timing of evaluation of response.
Acknowledgements
We thank Dr Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Nihon University School of Medicine and patient informed consent to treatments and procedures was obtained.
References
- Morita K, Takagi I, Watanabe M, et al. Relationship between the radiologic features of esophageal cancer and the local control by radiation therapy. Cancer 1985;55:2668-76. [Crossref] [PubMed]
- Yamada S, Takai Y, Nemoto K, et al. Prognostic factors in radiation-treated esophageal carcinoma. Acta Oncol 1992;31:563-7. [Crossref] [PubMed]
- Vyas RK, Baboo HA, Neema JP, et al. Carcinoma of the oesophagus: correlation of radiological appearance with response to radiotherapy. Indian J Chest Dis Allied Sci 1993;35:113-6. [PubMed]
- Japanese Society for Esophageal Disease. Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. 11th ed. Tokyo: Kanehara Co, 2015.
- Ishida K, Ando N, Yamamoto S, et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol 2004;34:615-9. [Crossref] [PubMed]
- Tahara M, Ohtsu A, Hironaka S, et al. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol 2005;35:316-23. [Crossref] [PubMed]
- Akiyama H. Surgery for carcinoma of the esophagus. Curr Probl Surg 1980;17:53-120. [Crossref] [PubMed]
- Olmsted WW, Lichtenstein JE, Hyams VJ. Polypoid epithelial malignancies of the esophagus. AJR Am J Roentgenol 1983;140:921-5. [Crossref] [PubMed]
- Sasajima K, Takai A, Taniguchi Y, et al. Polypoid squamous cell carcinoma of the esophagus. Cancer 1989;64:94-7. [Crossref] [PubMed]
- Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut 2004;53:925-30. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Yoshii T, Inokuchi Y, Sue S, et al. Analysis of endoscopic evaluation of primary response to CRT in advanced esophageal cancer. Prog Dig Endosc 2011;79:41-5. [Crossref]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Mori M, Mimori K, Sadanaga N, et al. Polypoid carcinoma of the esophagus. Jpn J Cancer Res 1994;85:1131-6. [Crossref] [PubMed]
- Nomiya T, Nemoto K, Miyaji H, et al. An Analysis of the Factors which Affect Prognosis and Formation of Macroscopic Type of Esophageal Carcinoma Jpn J Cancer Clin 2002;48:384-7. (in Japanese).
- Chino O, Makuuchi H, Shimada H, et al. Analysis of Macroscopic Tumor Type by Endoscopic Examination as well as the Invasive Depth of Protruding Esophageal Squamous Cell Carcinoma Stomach and Intestine 2013;48:321-35. (in Japanese).
- Chino O, Kijima H, Shimada H, et al. Clinicopathological studies of esophageal carcinosarcoma: analyses of its morphological characteristics using endoscopic, histological, and immunohistochemical procedures. Endoscopy 2000;32:706-11. [Crossref] [PubMed]
- Wu GX, Ituarte PH, Paz IB, et al. A Population-Based Examination of the Surgical Outcomes for Patients with Esophageal Sarcoma. Ann Surg Oncol 2015;22 Suppl 3:S1310-7. [Crossref] [PubMed]
- Takubo K, Mafune K, Tanaka Y, et al. Basaloid-squamous carcinoma of the esophagus with marked deposition of basement membrane substance. Acta Pathol Jpn 1991;41:59-64. [PubMed]
- Maebayashi T, Ishibashi N, Aizawa T, et al. A long-surviving patient with advanced esophageal basaloid squamous cell carcinoma treated only with radiotherapy: case report and literature review. BMC Gastroenterol 2017;17:151. [Crossref] [PubMed]
- Zenda S, Hironaka S, Taku K, et al. Optimal timing of endoscopic evaluation of the primary site of esophageal cancer after chemoradiotherapy or radiotherapy: a retrospective analysis. Dig Endosc 2009;21:245-51. [Crossref] [PubMed]


