Endoscopy in aortic valve repair: does it worth it?
Introduction
Aortic valve repair (AVR) is a technically challenging procedure, where attention must be paid to all anatomic and dynamic components of the aortic valve. These components include the sinotubular and aortoventricular junction, the dimensions of the aortic annulus, as well as the integrity and geometry of the aortic valve leaflets. Due to this complexity, many different techniques for AVR have been developed.
However, all these concepts inherit unsatisfactory conventional and quite subjective testing methods, such as a saline flush maneuver during cardioplegia-arrested heart. Moreover, final aortic valve function after repair can only be assessed by transoesophageal echocardiography (TOE) after weaning from cardio-pulmonary bypass. Insofar, an objective method to directly evaluate the repaired aortic valve during cross-clamp would be advantageous. Therefore, we evaluated the repaired aortic valve by the use of autoclavable endoscope, which was placed above the aortic valve while administering cardioplegia into the aortic root to create an artificial pressure during testing.
The aim of the present analysis was to evaluate the intraoperative results and potential modifications of AVR by the use of this additional tool of root endoscopy.
Methods
Study design
The present study was a retrospective single-center evaluation, including 66 patients undergoing aortic root endoscopy during AVR between 05/2014 and 03/2017. The present study obtained IRB-approval according to the Declaration of Helsinki. The primary study end-points were: need for Re-CPB after weaning from bypass and early postoperative aortic valve regurgitation at discharge. Secondary study end-points were: 30-day mortality and freedom from aortic regurgitation and reoperation during active follow-up.
Data collection and follow-up
Patient and operative demographics were recorded in a prospective institutional database and retrospectively extracted and evaluated. Perioperative deaths (30-day mortality) were tracked from the database or by active follow-up thereafter. Active follow-up was performed through December 2017 and was 100% complete. Active follow-up included: mortality, freedom from reoperation and degree of aortic regurgitation (last transthoracic echocardiography).
Operative technique
All operations were carried out through a standard (partial) median sternotomy and cardiopulmonary bypass was performed (CPB) with ascending aorta cannulation and single two-stage atrial cannula or selective bicaval cannula depending in the concomitant procedure. A cannula was inserted at the junction of the right superior pulmonary vein for venting of the left ventricle. Moderate hypothermia (28–32 °C) was obtained. Myocardial protection was achieved by antegrade and optional retrograde crystalloid cardioplegic arrest (Custodiol®, Dr. Köhler Chemie, Bensheim, Germany) and additional topic cooling. A hockey-stick aortotomy was performed. AVR was performed in patients presenting aortic stenosis, aortic valve regurgitation or combination of both using different techniques, such as aortic valve re-implantation, aortic valve re-construction by using either autologous or tissue engineered pericardium, as well as different complex aortic cusp repair techniques. When indicated, replacement of the ascending aorta was also performed. During cardioplegia-arrested heart after completed AVR, the operative result was directly evaluated: This was achieved by placing an autoclavable video-scope (rigid 0° scope, 5 mm diameter, WA50020B, Endo-Eye®, Olympus, Tokyo, Japan) (Figure 1) into the aortic root while the proximal aorta or the aortic vascular graft was clamped and crystalloid cardioplegia was administered to create an artificial blood pressure (~100 mmHg) within the aortic root. Moreover, while cardioplegia was administered, the left ventricle was simultaneously vented (Figure 2).
Statistics
Continuous data were expressed as mean ± standard deviation (SD), and categorical data were expressed as percentages or frequencies. Survival curves and freedom from reoperation were generated with the Kaplan-Meier method. All statistical analyses were performed with the SPSS software (version 22.0, IBM Crop., Armonk, NY, USA). The authors had full access to the data and take full responsibility for the integrity of the data.
Results
Patient population
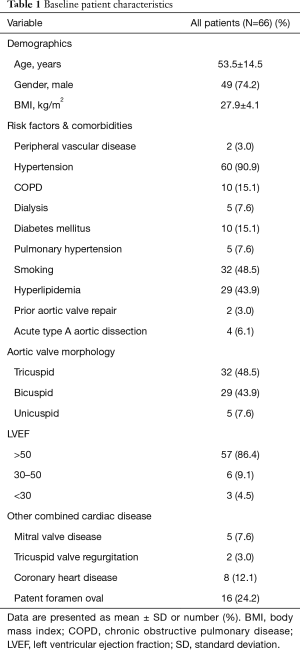
Mean age of the total group was 53.5±14.5 years (range, 22–76 years), 74.2% (n=49) were male, and 73% presented with New York Heart Association (NYHA) class ≥ III. The pre-operative underlying pathology of the aortic valve was stenosis in 9.1% (6 patients), regurgitation in 83.3% (55 patients, of whom 4 presented with acute type A aortic dissection) and combination of both in 7.6% (5 patients). Demographics are presented in Table 1. Most of the patients (98.5%) presented with additional pathology of the ascending aorta (aneurysm or dissection).
Full table
Operative outcomes and primary study endpoints
AVR was performed via aortic valve re-implantation (David procedure) in 45 (68.2%) patients, replacement of all three cusps using either autologous or tissue-engineered pericardium in 13 (19.7%) patients or complex aortic valve cusp repair in 8 (12.1%) patients, of whom seven received concomitant replacement of the ascending aorta. In total, replacement of the ascending aorta was performed in 97% of the patients. Concomitant procedures were performed in 68.2% of the patients: concomitant coronary bypass surgery in 8 (12.1%) patients, mitral valve repair in 5 (7.6%) patients, tricuspid valve repair in 2 (3.0%) patients, closure of patent foramen ovale in 16 (24.2%) patients. In 10 (15.1%) patients, various concomitant procedures were performed, such as subvalvular myectomy, left atrial appendage closure, or a MAZE procedure. Various cusp repair techniques (shaving, plication sutures, decalcification, bicuspidalisation, tricuspidalisation, raphe resection, triangular or complete cusp resection for at least one cusp) were performed in 58 patients. Table 2 summarizes all operative outcomes.
Full table
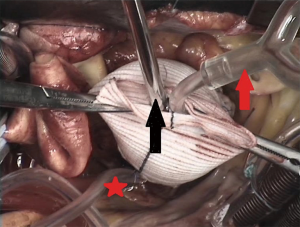
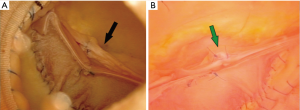
Apart of 8 patients, second time cross-clamp was avoided in most [58] of the patients whereas extra plication was applied to the valve in 13 patients (e.g., Figure 3) after aortic valve evaluation using pressurized aortic root endoscopy. Out of these 8 patients who needed re-clamping, 2 patients had bleeding that could not stasis without arrested heart and the other 6 patients due to residual regurgitation that was underestimated during endoscopy. This residual aortic regurgitation was treated by resection of the non-coronary sinus in 1 patient (Figure 4), replacement of an aortic cusp using autologous pericardium in another patient, and additional plication was endorsed for the other 4 patients. After final weaning from CPB, intraoperative TOE control revealed no aortic regurgitation was observed in 44 (66.7%) patients, and only trivial aortic regurgitation in 22 (33.3%) patients.
Secondary study endpoints
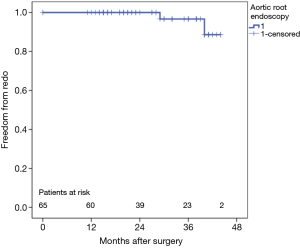
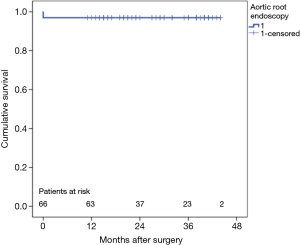
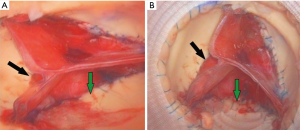
Thirty-day mortality was 3% (n=2) due to stunning heart caused by severe left ventricular hypertrophy in one patient. The other patient experienced a massive cerebral bleeding after aortic valve re-implantation after acute type A aortic dissection. During follow-up (mean 28±10 months) no deaths occurred, but two patients showed recurrent aortic regurgitation. The first patient experienced a suture dehiscence between the autologous pericardial patches 7 months postoperatively. This patient already had a second cross-clamp during index surgery as mentioned above. During redo-surgery, the aortic valve was replaced by a mechanical substitute. The second patient, who showed a unicuspid aortic valve during index procedure and was initially treated by bicuspidalisation presented with acute cusp perforation 1 week postoperatively. This patient was successfully treated by replacement of the perforated cusp with tissue-engineered pericardium. Estimated overall freedom from reoperation (Figure 5) and cumulative survival (Figure 6) are illustrated with Kaplan-Meier curves. Finally, Table 3 summarizes the echocardiographic assessment of the aortic regurgitation intraoperatively, at discharge as well as at the last follow-up.

Full table
Discussion
The main findings of this study are: (I) aortic valve endoscopy is a feasible tool, which can be easily applied to evaluate AVR before weaning of the CPB; (II) despite this helpful tool, unsuspected factors or under-estimated aortic regurgitation during endoscopic evaluation might cause second cross clamping; (III) early results of AVR with endoscopic guidance were good with a 30-day mortality of 3%; (IV) during follow-up, however, two patients needed reoperation due to severe aortic regurgitation, additional two patients have mild to moderate regurgitation and only none to trivial aortic regurgitation was observed for the rest of patients.
Currently, AVR or aortic sparing operations, such as aortic valve re-implantation [David’s procedure (1), aortic root remodeling (2), various cusp repair techniques e.g., Ozaki’s method (3)] are gaining increased interest. To test the competence of the repaired aortic valve, usually subjective test methods during cardioplegia-arrested heart in the non-beating situation are used, like saline flushing or excessive venting. Correct aortic valve function, however, depends not only on leaflet function, but also on other components, like the sinotubular junction, the aortoventricular junction as well as the aortic sinuses. The perfect combination of these components, during the dynamic situation of the cardiac cycle will lead to a competent aortic valve. This situation however, can be only achieved, when the heart is weaned from CPB. Then, TOE offers a detailed view of the repaired aortic valve, and competence can be evaluated, gradients can be measured and the coaptation height and length of the leaflets can the evaluated. Insofar, an objective method to directly evaluate the repaired aortic valve during cross clamping would be advantageous. Therefore, an idea was suggested to visualize the aortic valve after repair in the diastolic position and under “physiologic” conditions, making the evaluation of the symmetry of the leaflets most similar to reality. Of course, our concept represents just an approximation towards physiological conditions, but it can be seen as a first step.
Early attempts to use an endoscope in the diagnosis or management of cardiac/vessel disease were discouraging due to visualization problems (4-6). These concepts disappeared with the introduction and possibility of open-heart surgery. Nowadays however, technology evolved and there is an increased demand for minimally invasive surgery. Therefore, new imaging options and modalities play a more and more important role in various surgical specialties. Duran first described the concept aortic valve endoscopy in 1991. He reported the use of a so-called “valvoscope” in three patients. In these patients, the method of aortic valve visualization was used to examine the proper leaflet length of left coronary neo-cusps. According to the visualized prolapse of this left coronary leaflet, Duran resected a few millimeters of the free edge to prevent potential coronary ischemia caused by the excessive leaflet material (7). Thereafter, Itoh and colleges rediscovered the concept of using an endoscope in 1997. They reported the successful and helpful use of intraoperative endoscopy in valve sparing operations to reduce the postoperative incidence of aortic regurgitation (8,9). They noted the potential indication of intraoperative endoscopy to evaluate AVR, as well as experimentally attempt to clarify the role of dilatation of the sinotubular junction as one cause of aortic regurgitation (10). Their results were encouraging and similar to our very primary experience with root endoscopy (11).
The present study presents the early to mid-term results of 66 consecutive patients undergoing successful AVR in our center in which intraoperative aortic root endoscopy was applied. One of the main finding of the present study is, that intraoperative endoscopy during cardioplegia-arrested heart led to additional cusp plication in 13 patients and avoided second time cross-clamp and faster weaning from CPB in most [58] of the cases. However, 2nd cross-clamp was needed in 8 patients, 2 due to bleeding that could not be stasis without arrested heart, in the other 6 patients; a residual aortic valve regurgitation was defined with the TOE. This residual regurgitation needed additional correction and was treated by resection of the non-coronary sinus in 1 patient (Figure 4), replacement of an aortic cusp using autologous pericardium in another patient, and additional plication was endorsed for the other 4 patients. Of course, the pressurized plastic bag containing the cardioplegia is not able to produce physiological conditions within the aortic root and moreover, obviously only the diastolic phase without any leaflet motion can be observed, in addition to the valve evaluation obtained by the endoscope are still quite subjective and could be underestimated from the surgeon himself due to the learning-curve of using endoscopy as well. Nevertheless, in our opinion, the particular advantages of this technique should be recognized: the technique itself is easy and fast to apply, very cost-effective, as all the components used were autoclavable and offers a “real-time” view on the pressurized aortic root after repair, which adds a certain amount of confidence to the surgeon. According to our “mid-term” results, the vast majority of patients present none to trace aortic regurgitation during follow-up, and only 2 patients had to be-operated due to residual/recurrent aortic regurgitation.
Due to our best knowledge today, there was only one study, which described the successful use of intraoperative endoscopic guidance in a small cohort of patients (n=17). In addition to our study, Ohtsubo and colleagues also inserted their endoscope directly after placing the aortic clamp and they evaluated the pathology prior to repair. They reported that 8 patients underwent aortic remodeling, 4 patients were treated by aortic valve re-implantation, and the remaining 5 patients received a valved-conduit. Three patients who underwent reimplantation procedure required late valve replacement for late progression of aortic regurgitation. They concluded that (I) aortic root endoscopy successfully reduced postoperative aortic regurgitation, (II) that root endoscopy might help to clarify the indications, but also limitations of valve-sparing root operations, and (III) that an endoscopically-judged minor prolapse might be a predictor for long-term results (9).
Limitations
The present study was performed at a single tertiary care medical center and was retrospective. The generalization of our findings may not extend to all centers worldwide, as AVR is a complex procedure and comes with a “learning-curve”, which is also true for root endoscopy. Moreover, the application and interpretation of results, obtained by an endoscope are still quite subjective and were obtained under pseudo-“physiological” conditions and could be underestimated from the surgeon himself as seen in 6 patients of our series due to the learning-cure of using endoscopy as well. Further evaluation in larger cohorts is warranted.
Conclusions
In conclusion, aortic root endoscopy is an easy to apply, and helpful tool to evaluate the repaired aortic valve before weaning from bypass. This technique might have the potential to lower the incidence of second cross clamping during AVR. Moreover, aortic root endoscopy might be also used for educational or training issues. To summarize, in our institution root endoscopy is routinely used, even in situations like acute aortic type A dissections, as we strongly believe in this helpful technique, as it is a further piece in the puzzle to get perfect results after AVR.
Acknowledgements
The authors are sincerely grateful to Miriam Radu, Andreas Sander (Institute of Quality Controlling, West-German Heart and Vascular Center, University Hospital Essen, Essen, Germany) for their generous effort and support in the data review to finish this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study obtained IRB-approval (18-8199-BO) according to the Declaration of Helsinki.
References
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [PubMed]
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8. [PubMed]
- Ozaki S, Kawase I, Yamashita H, et al. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg 2014;147:301-6. [Crossref] [PubMed]
- Rhea L, Walker IC, Cutler EC. The surgical treatment of mitral stenosis: experimental and clinical studies. Arch Surg 1924;9:689-90. [Crossref]
- Allen DS, Graham EA. Intracardiac surgery—a new method. Am Med Ass 1922;79:1028. [Crossref]
- Butterworth RF. A new operating cardioscope. J Thorac Surg 1951;22:319-22. [PubMed]
- Duran C, Kumar N, Gometza B, et al. Indications and limitations of aortic valve reconstruction. Ann Thorac Surg 1991;52:447-53; discussion 453-4. [Crossref] [PubMed]
- Itoh T, Ohtsubo S, Furukawa K, et al. Aortic root endoscopy in valve-sparing operations. J Thorac Cardiovasc Surg 1997;114:141-2. [Crossref] [PubMed]
- Ohtsubo S, Itoh T, Natsuaki M, et al. Successful valve-sparing in aortic root reconstruction under endoscopic guidance. Eur J Cardiothorac Surg 2000;17:420-5. [Crossref] [PubMed]
- Furukawa K, Ohteki H, Cao ZL, et al. Does dilatation of the sinotubular junction cause aortic regurgitation? Ann Thorac Surg 1999;68:949-53; discussion 953-4. [Crossref] [PubMed]
- Tsagakis K, Benedik J, El Khoury G, et al. Aortic valve repair: Intraoperative evaluation of valve geometry by angioscopy. J Thorac Cardiovasc Surg 2015;149:1666-8. [Crossref] [PubMed]